CpG incorporated DNA microparticles for elevated immune stimulation for antigen presenting cells
- PMID: 35540407
- PMCID: PMC9078369
- DOI: 10.1039/c7ra13293j
CpG incorporated DNA microparticles for elevated immune stimulation for antigen presenting cells
Erratum in
-
Correction: CpG incorporated DNA microparticles for elevated immune stimulation for antigen presenting cells.RSC Adv. 2019 Feb 22;9(11):6395. doi: 10.1039/c9ra90013f. eCollection 2019 Feb 18. RSC Adv. 2019. PMID: 35532418 Free PMC article.
Abstract
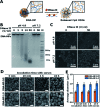
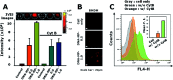
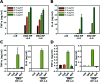
As emerging evidence supports the immune stimulating capability of the CpG oligodeoxynucleotides (ODN), CpG-based adjuvants have been widely used. For efficient induction of immune responses, current issues affecting the use of nucleic acid-based adjuvants, e.g. stability in physiological conditions, delivery to immune cells, and successful release within the phagolysosome, should be addressed. Here, we present CpG-based DNA microparticles (DNA-MPs) fabricated by complementary rolling circle amplification (cRCA) as adjuvants for enhancing immune response and production of selective antibody production. Using cRCA method, the sizes of CpG-based DNA-MPs were finely controlled (0.5 and 1 μm) with superior and provided mismatched single stranded form of CpG ODN region for specific cleavage site by DNase II within the phagolysosome. Fabricated CpG-based 1 μm DNA-MPs (DNA-MP-1.0) were successfully internalized into primary macrophages and macrophage cell line (RAW264.7 cells), and elicited superior cytokine production e.g. TNF-α and IL-6, compared to conventional CpG ODNs. After in vivo administration of DNA-MP-1.0 with model antigen ovalbumin (OVA), significantly elevated OVA-specific antibody production was observed. With this in mind, DNA-MP-1.0 could serve as a novel type of adjuvant for the activation of macrophages and the following production of selective antibodies without any noticeable toxicity in vitro and in vivo.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles.J Immunother. 2007 Jul-Aug;30(5):469-78. doi: 10.1097/CJI.0b013e31802fd8c6. J Immunother. 2007. PMID: 17589287
-
Carbonate Apatite Nanoparticles Act as Potent Vaccine Adjuvant Delivery Vehicles by Enhancing Cytokine Production Induced by Encapsulated Cytosine-Phosphate-Guanine Oligodeoxynucleotides.Front Immunol. 2018 Apr 18;9:783. doi: 10.3389/fimmu.2018.00783. eCollection 2018. Front Immunol. 2018. PMID: 29720976 Free PMC article.
-
Immunostimulatory Properties of Lipid Modified CpG Oligonucleotides.Mol Pharm. 2017 Aug 7;14(8):2815-2823. doi: 10.1021/acs.molpharmaceut.7b00335. Epub 2017 Jul 24. Mol Pharm. 2017. PMID: 28686452
-
Co-encapsulated CpG oligodeoxynucleotides and ovalbumin in PLGA microparticles; an in vitro and in vivo study.J Pharm Pharm Sci. 2014;17(4):541-53. doi: 10.18433/j33892. J Pharm Pharm Sci. 2014. PMID: 25579433
-
Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system.Int J Nanomedicine. 2012;7:2181-95. doi: 10.2147/IJN.S30197. Epub 2012 Apr 27. Int J Nanomedicine. 2012. PMID: 22619554 Free PMC article. Review.
Cited by
-
Small Molecule NF-κB Inhibitors as Immune Potentiators for Enhancement of Vaccine Adjuvants.Front Immunol. 2020 Sep 25;11:511513. doi: 10.3389/fimmu.2020.511513. eCollection 2020. Front Immunol. 2020. PMID: 33072085 Free PMC article.
-
Increased vaccine tolerability and protection via NF-κB modulation.Sci Adv. 2020 Sep 9;6(37):eaaz8700. doi: 10.1126/sciadv.aaz8700. Print 2020 Sep. Sci Adv. 2020. PMID: 32917696 Free PMC article.
-
Injectable Micro-Hydrogel for DNA Delivery: A Promising Therapeutic Platform.J Funct Biomater. 2024 Mar 1;15(3):59. doi: 10.3390/jfb15030059. J Funct Biomater. 2024. PMID: 38535252 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

