One-step preparation of a novel SrCO3/g-C3N4 nano-composite and its application in selective adsorption of crystal violet
- PMID: 35540413
- PMCID: PMC9078232
- DOI: 10.1039/c7ra11565b
One-step preparation of a novel SrCO3/g-C3N4 nano-composite and its application in selective adsorption of crystal violet
Abstract
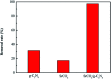
A novel kind of nanoparticle SrCO3/g-C3N4 was prepared using strontium carbonate (SrCO3) and melamine (C3H6N6) as raw materials via one-step calcination. The formation of SrCO3/g-C3N4 was confirmed from the X-ray diffraction (XRD), Fourier transform infrared spectra (FT-IR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Brunauer-Emmett-Teller (BET) and X-ray photoelectron spectroscopy (XPS) analysis. Its selective adsorption performance was evaluated towards crystal violet (CV), rhodamine B (RhB) and methylene blue (MB). The results showed that the SrCO3/g-C3N4 had selective adsorption ability of CV. Furthermore, adsorption measurements of CV were conducted to investigate the influences of contact time, initial concentration, initial dye solution pH value and adsorbent dosage. The maximum removal rate of CV was 98.56% when the initial concentration was 1600 mg L-1. The kinetic study indicated the adsorption of CV followed the pseudo-second-second model well. The adsorption efficiency of SrCO3/g-C3N4 was greater (97.46%) than that of g-C3N4 (31.30%) and SrCO3 (17.30%). It could be deduced that the synergistic effect of conjugation interaction of g-C3N4 and the electrostatic attraction of SrCO3 might be the main driving force for the superb adsorption of CV.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures














Similar articles
-
Facile synthesis of high-efficiency magnetic graphitic carbon nitride adsorbents for the selective removal of hazardous anionic dyes in wastewater.Dalton Trans. 2022 Oct 25;51(41):15842-15853. doi: 10.1039/d2dt02320b. Dalton Trans. 2022. PMID: 36190136
-
[Preparation of melamine-functionalized porous organic polymer and its adsorption properties for methyl orange].Se Pu. 2021 Sep;39(9):998-1005. doi: 10.3724/SP.J.1123.2021.06016. Se Pu. 2021. PMID: 34486839 Free PMC article. Chinese.
-
Fabrication of Nano Iron Oxide-Modified Biochar from Co-Hydrothermal Carbonization of Microalgae and Fe(II) Salt for Efficient Removal of Rhodamine B.Nanomaterials (Basel). 2022 Jul 1;12(13):2271. doi: 10.3390/nano12132271. Nanomaterials (Basel). 2022. PMID: 35808107 Free PMC article.
-
Preparation of new adsorbent-supported Fe/Ni particles for the removal of crystal violet and methylene blue by a heterogeneous Fenton-like reaction.RSC Adv. 2019 Jul 22;9(39):22513-22522. doi: 10.1039/c9ra04710g. eCollection 2019 Jul 17. RSC Adv. 2019. PMID: 35519486 Free PMC article.
-
Investigation of Mesoporous Graphitic Carbon Nitride as the Adsorbent to Remove Ni (II) Ions.Water Environ Res. 2016 Apr;88(4):318-24. doi: 10.2175/106143015X14212658613398. Water Environ Res. 2016. PMID: 27131055
Cited by
-
Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis.Molecules. 2021 Jul 23;26(15):4451. doi: 10.3390/molecules26154451. Molecules. 2021. PMID: 34361602 Free PMC article.
-
Simultaneous Analysis of 53 Pesticides in Safflower (Carthamus tinctorius L.) by Using LC-MS/MS Coupled with a Modified QuEChERS Technique.Toxics. 2023 Jun 16;11(6):537. doi: 10.3390/toxics11060537. Toxics. 2023. PMID: 37368637 Free PMC article.
-
ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application.ACS Omega. 2020 Feb 18;5(8):3828-3838. doi: 10.1021/acsomega.9b02688. eCollection 2020 Mar 3. ACS Omega. 2020. PMID: 32149209 Free PMC article.
-
Influence of Novel SrTiO3/MnO2 Hybrid Nanoparticles on Poly(methyl methacrylate) Thermal and Mechanical Behavior.Polymers (Basel). 2024 Jan 19;16(2):278. doi: 10.3390/polym16020278. Polymers (Basel). 2024. PMID: 38276687 Free PMC article.
References
-
- Ding F. Yang D. Tong Z. W. Nan Y. H. Wang Y. J. Zou X. Y. Jiang Z. Y. Graphitic carbon nitride-based nanocomposites as visible-light driven photocatalysts for environmental purification. Environ. Sci.: Nano. 2017;4:1455–1469. doi: 10.1039/C7EN00255F. - DOI
-
- Shi A. Y. Li H. H. Yin S. Liu B. Zhang J. C. Wang Y. H. Effect of conjugation degree and delocalized π-system on the photocatalytic activity of single layer g-C3N4. Appl. Catal., B. 2017;218:137–146. doi: 10.1016/j.apcatb.2017.06.017. - DOI
-
- Wu M. Yan J. M. Zhang X. W. Zhao M. Synthesis of g-C3N4 with heating acetic acid treated melamine and its photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2015;354:196–200. doi: 10.1016/j.apsusc.2015.01.132. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

