Effect of fluorination of naphthalene diimide-benzothiadiazole copolymers on ambipolar behavior in field-effect transistors
- PMID: 35540535
- PMCID: PMC9080245
- DOI: 10.1039/c8ra02915f
Effect of fluorination of naphthalene diimide-benzothiadiazole copolymers on ambipolar behavior in field-effect transistors
Abstract
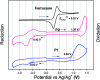
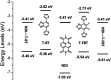
Two naphthalene diimide (NDI)-benzothiadiazole (BT) based conjugated polymers with high molecular weight, P1 and P2, were synthesized by introducing F atoms to modulate the electron-donating ability of the BT moiety. 3-Decyl-pentadecyl branched alkyl side chains were employed and expected to improve the molecular organization and device performance. Both polymers have excellent solubility in common organic solvents. UV-vis-NIR absorption and cyclic voltammetry indicate that the maximum absorption wavelength of P2 is blue-shifted and the HOMO energy level of P2 is decreased in comparison with P1. Two dimensional wide angle X-ray scattering of thin films revealed a similar organization of both polymers. A less balanced transport in field-effect transistors with increased electron mobility of 0.258 cm2 V-1 s-1 and lowered hole transport of 2.4 × 10-3 cm2 V-1 s-1 was found for P2. Polymer devices of P1 exhibited a balanced ambipolar transport, with a hole mobility of 0.073 cm2 V-1 s-1 and electron mobility of 0.086 cm2 V-1 s-1.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
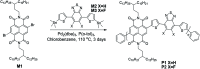



References
LinkOut - more resources
Full Text Sources
Miscellaneous

