Strong, tough and mechanically self-recoverable poly(vinyl alcohol)/alginate dual-physical double-network hydrogels with large cross-link density contrast
- PMID: 35540543
- PMCID: PMC9080324
- DOI: 10.1039/c8ra01302k
Strong, tough and mechanically self-recoverable poly(vinyl alcohol)/alginate dual-physical double-network hydrogels with large cross-link density contrast
Abstract
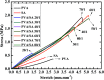
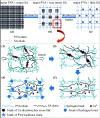
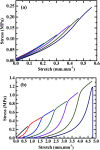
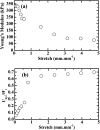
Strong and tough poly(vinyl alcohol) (PVA)/alginate hydrogen-bonded-ionic dual-physical double-network (DN) hydrogels have been successfully prepared by a facile route of a freeze-thaw (25-25-25 °C) cycle followed by concentrated (1.0 mol L-1 of) aqueous-Ca2+ immersion of PVA/Na alginate (SA) mixed aqueous solutions. It was found that, at mole ratios of the PVA- to SA repeat units of 20/1 to 80/1, the DN gels likely evolved a semi-interpenetrating polymer network (IPN) morphology of rigid alginate networks dispersed in while interlocking with ductile PVA network to accomplish DN synergy that gave their high strength and toughness, where the high alginate rigidity originated probably from its dense cross-link induced syneresis and dispersion along crosslink-defective voids to result in little internal stress concentration. Tentatively mechanistically, as the 20/1-80/1 DN gels were stretched steadily, their mechanical response was gradually differentiated into distinct synergistic states: the sparsely hydrogen-bonded PVA served as a ductile matrix to bear small fractions of the established stresses at its large elongations; whereas the densely ionically (i.e. Ca2+) cross-linked alginate functioned as a rigid skeleton to sustain the remaining larger stresses upon its smaller local strains. Promisingly, this ductile-rigid matrix-skeleton synergistic mechanism of semi-IPN morphology may be universally extended to all A/B DN hydrogels of large A-B rigidity (or cross-link density) contrast, whether the cross-link nature of network(s) A or B is covalent, ionic, hydrogen bonded or van der Waals interacted. The strong and tough DN gels also displayed satisfactory self-recovery of viscoelastic behaviour, in that their Young's modulus and dissipated energy in the uniaxial tensile mode and dynamic storage and loss moduli in the oscillatory shear mode all recovered significantly from non-linear viscoelastic regimes despite different degrees of failure to revert to (quasi)linear viscoelasticity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Tough, stretchable and compressive alginate-based hydrogels achieved by non-covalent interactions.RSC Adv. 2020 Jun 22;10(40):23592-23606. doi: 10.1039/d0ra03733h. eCollection 2020 Jun 19. RSC Adv. 2020. PMID: 35517309 Free PMC article.
-
Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels.Acta Biomater. 2022 Jan 15;138:301-312. doi: 10.1016/j.actbio.2021.10.044. Epub 2021 Oct 29. Acta Biomater. 2022. PMID: 34757233
-
Bioinspired fully physically cross-linked double network hydrogels with a robust, tough and self-healing structure.Mater Sci Eng C Mater Biol Appl. 2017 May 1;74:374-381. doi: 10.1016/j.msec.2016.12.026. Epub 2016 Dec 7. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28254307
-
Sodium alginate/polyvinyl alcohol semi-interpenetrating hydrogels reinforced with PEG-grafted-graphene oxide.Int J Biol Macromol. 2024 Apr;263(Pt 2):130258. doi: 10.1016/j.ijbiomac.2024.130258. Epub 2024 Feb 29. Int J Biol Macromol. 2024. PMID: 38423903
-
Double-Network Tough Hydrogels: A Brief Review on Achievements and Challenges.Gels. 2022 Apr 18;8(4):247. doi: 10.3390/gels8040247. Gels. 2022. PMID: 35448148 Free PMC article. Review.
Cited by
-
Antimicrobial effects of hydroxyapatite mosaicked polyvinyl alcohol-alginate semi-interpenetrating hydrogel-loaded with ethanolic extract of Glycyrrhiza glabra against oral pathogens.Prog Biomater. 2022 Dec;11(4):373-383. doi: 10.1007/s40204-022-00199-2. Epub 2022 Aug 15. Prog Biomater. 2022. PMID: 35969367 Free PMC article.
-
Tough Hydrogels for Load-Bearing Applications.Adv Sci (Weinh). 2024 Mar;11(12):e2307404. doi: 10.1002/advs.202307404. Epub 2024 Jan 15. Adv Sci (Weinh). 2024. PMID: 38225751 Free PMC article. Review.
-
Network of cyano-p-aramid nanofibres creates ultrastiff and water-rich hydrospongels.Nat Mater. 2024 Mar;23(3):414-423. doi: 10.1038/s41563-023-01760-5. Epub 2024 Jan 5. Nat Mater. 2024. PMID: 38182810
-
Tunable Alginate-Polyvinyl Alcohol Bioinks for 3D Printing in Cartilage Tissue Engineering.Gels. 2024 Dec 14;10(12):829. doi: 10.3390/gels10120829. Gels. 2024. PMID: 39727587 Free PMC article.
-
Dynamic Hydrogel-Based Strategy for Traumatic Brain Injury Modeling and Therapy.CNS Neurosci Ther. 2025 Jan;31(1):e70148. doi: 10.1111/cns.70148. CNS Neurosci Ther. 2025. PMID: 39788897 Free PMC article. Review.
References
-
- Kirchmajer D. M., PhD thesis, University of Wollongong, Wollongong, Australia, 2013
-
- Jaiswal M. Dinda A. K. Gupta A. Koul V. Biomed. Mater. 2010;5:9595. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

