Latest perspectives of orally bioavailable 2,4-diarylaminopyrimidine analogues (DAAPalogues) as anaplastic lymphoma kinase inhibitors: discovery and clinical developments
- PMID: 35540549
- PMCID: PMC9080316
- DOI: 10.1039/c8ra01934g
Latest perspectives of orally bioavailable 2,4-diarylaminopyrimidine analogues (DAAPalogues) as anaplastic lymphoma kinase inhibitors: discovery and clinical developments
Abstract
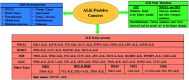
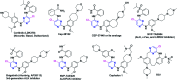
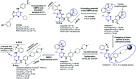
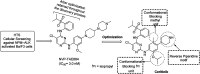
The course of anaplastic lymphoma kinase (ALK)-rearranged non-small-cell lung cancer (NSCLC) therapy has improved impressively. The Food and Drug Administration (FDA) has approved crizotinib (Xalkori, Pfizer) as a first-in-class tyrosine kinase inhibitor (TKI) that demonstrated a substantial objective response rate (ORR) and remarkable progression-free survival (PFS). However, acquired resistance to crizotinib is still a major concern especially as the central nervous system (CNS) remains the most common sites of relapse. To combat disease resistance, limited PFS and poor CNS exposure exhibited by crizotinib (Xalkori, Pfizer) led to the discovery of numerous next generation ALK-TKIs and surprisingly most of them are 2,4-Diarylaminopyrimidine Analogues (DAAPalogues). To date, DAAPalogues have been investigated extensively to display their superior potency against numerous kinase targets especially ALK/ROS1. This review describes hit-to-drug evolution strategies, activity spectra, milestones related to medicinal chemistry discovery efforts and scalable synthetic pathways of clinically emerging DAAPalouges which are either progressing as investigational or preclinical candidates. In addition, the significance of DAAPalogues to treat the patients with ALK+-NSCLC in clinical settings has been detailed. This review is beneficial for medicinal chemists and researchers contributing to discovering ALK-TKIs to overcome existing issues related to DAAPalouges in the drug discovery process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors also declare no conflicts of interest.
Figures




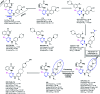
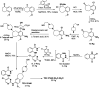







Similar articles
-
Will the Requirement by the US FDA to Simultaneously Co-Develop Companion Diagnostics (CDx) Delay the Approval of Receptor Tyrosine Kinase Inhibitors for RTK-Rearranged (ROS1-, RET-, AXL-, PDGFR-α-, NTRK1-) Non-Small Cell Lung Cancer Globally?Front Oncol. 2014 Apr 1;4:58. doi: 10.3389/fonc.2014.00058. eCollection 2014. Front Oncol. 2014. PMID: 24744988 Free PMC article. Review.
-
P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer.EBioMedicine. 2015 Dec 12;3:54-66. doi: 10.1016/j.ebiom.2015.12.009. eCollection 2016 Jan. EBioMedicine. 2015. PMID: 26870817 Free PMC article.
-
Anaplastic lymphoma kinase tyrosine kinase inhibitors in non-small cell lung cancer.Transl Cancer Res. 2019 Jan;8(Suppl 1):S48-S54. doi: 10.21037/tcr.2018.10.23. Transl Cancer Res. 2019. PMID: 35117063 Free PMC article. Review.
-
Anaplastic lymphoma kinase inhibitors in phase I and phase II clinical trials for non-small cell lung cancer.Expert Opin Investig Drugs. 2017 Jun;26(6):713-722. doi: 10.1080/13543784.2017.1324572. Epub 2017 May 18. Expert Opin Investig Drugs. 2017. PMID: 28463570 Review.
-
Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial.Lancet Oncol. 2017 Dec;18(12):1590-1599. doi: 10.1016/S1470-2045(17)30680-0. Epub 2017 Oct 23. Lancet Oncol. 2017. PMID: 29074098 Free PMC article. Clinical Trial.
Cited by
-
Selected β2-, β3- and β2,3-Amino Acid Heterocyclic Derivatives and Their Biological Perspective.Molecules. 2021 Jan 15;26(2):438. doi: 10.3390/molecules26020438. Molecules. 2021. PMID: 33467741 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

