Guanine deaminase provides evidence of the increased caffeine content during the piling process of pu'erh tea
- PMID: 35540571
- PMCID: PMC9075041
- DOI: 10.1039/c9ra05655f
Guanine deaminase provides evidence of the increased caffeine content during the piling process of pu'erh tea
Abstract
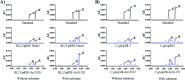
Wet piling is a key process for producing pu'erh tea because various components change under the action of microorganisms. Among these components, caffeine content is increased. Evidence has indicated a salvage pathway for caffeine biosynthesis in microbes, in which xanthine is methylated in the order of N-3 → N-1 → N-7. In addition, guanine can be used to synthesize xanthine through guanine deaminase (EC: 3.5.4.3). In this study, we investigated the variation in caffeine content during piling fermentation with supplementary guanine, 15N-labeled guanine and xanthine. We cloned the guanine deaminase gene (GUD1) from Saccharomyces cerevisiae (one dominant strain in piling fermentation). The results revealed that [15N]xanthine could be synthesized from [15N]guanine, and [15N]caffeine was also detected during piling with supplementary [15N]xanthine. Furthermore, ScGUD1 could catalyze the conversion of guanine to xanthine, which is likely to be methylated for caffeine synthesis under microorganism action. The obtained results revealed the mechanism underlying the increased caffeine content during piling of pu'erh tea.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

