DABCO bond cleavage for the synthesis of piperazine derivatives
- PMID: 35540608
- PMCID: PMC9075132
- DOI: 10.1039/c9ra07870c
DABCO bond cleavage for the synthesis of piperazine derivatives
Abstract
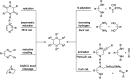
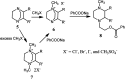
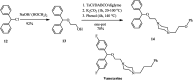
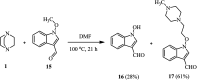
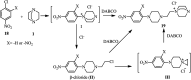
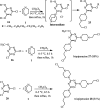
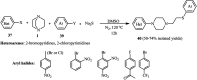
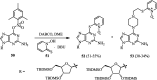
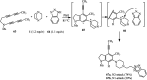
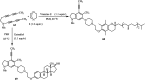
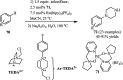
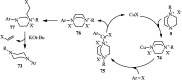
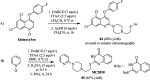
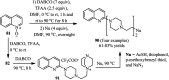
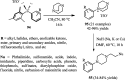
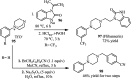
The applications of DABCO (1,4-diazabicyclo[2.2.2]octane) in the synthesis of piperazine derivatives including biologically active compounds via C-N bond cleavage are investigated in this review. Different reagents such as alkyl halides, aryl(heteroary) halides, carboxylic acids, diaryliodonium salts, tosyl halides, activated alkynes, benzynes etc. were applied for the preparation of the corresponding quaternary ammonium salts of DABCO, which are very good electrophiles for various nucleophiles such as phenols, thiophenols, thiols, alcohols, aliphatic and aromatic amines, sulfinates, phthalimide, indoles, NaN3, triazole and terazoles, NaCN, enols and enolates, halides, carboxylic acid salts etc. Besides preactivated DABCO salts, the in situ activation of DABCO in multicomponent reactions is also an efficient tactic in synthetic organic chemistry for the diversity oriented synthesis of drug-like piperazine derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


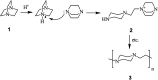



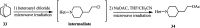
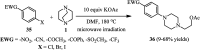


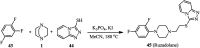
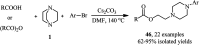

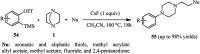

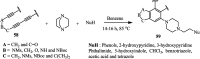
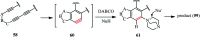





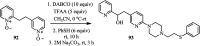
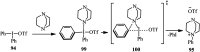

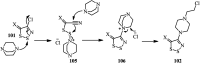
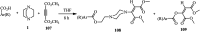






Similar articles
-
One-pot four-component synthesis of novel isothiourea-ethylene-tethered-piperazine derivatives.RSC Adv. 2023 Nov 7;13(46):32772-32777. doi: 10.1039/d3ra06678a. eCollection 2023 Oct 31. RSC Adv. 2023. PMID: 37942451 Free PMC article.
-
Ring-opening reactions of 1,4-diazabicyclo[2.2.2]octane (DABCO) derived quaternary ammonium salts with phenols and related nucleophiles.Org Biomol Chem. 2012 Feb 14;10(6):1300-10. doi: 10.1039/c1ob06676e. Epub 2012 Jan 4. Org Biomol Chem. 2012. PMID: 22215139
-
DABSO as a SO2 gas surrogate in the synthesis of organic structures.Org Biomol Chem. 2022 Mar 16;20(11):2149-2163. doi: 10.1039/d1ob02199k. Org Biomol Chem. 2022. PMID: 35170616 Review.
-
N-Alkylated 1,4-Diazabicyclo[2.2.2]octane-Polyethylene Glycol Melt as Deep Eutectic Solvent for the Synthesis of Fisher Indoles and 1H-Tetrazoles.ACS Omega. 2017 Jun 22;2(6):2891-2900. doi: 10.1021/acsomega.7b00618. eCollection 2017 Jun 30. ACS Omega. 2017. PMID: 31457624 Free PMC article.
-
Recent Advances in the Pd-Catalyzed Coupling of Arylhydrazines and Ammonium Salts via C-N Bond Cleavage.Chem Rec. 2021 Dec;21(12):3442-3457. doi: 10.1002/tcr.202100089. Epub 2021 Jun 26. Chem Rec. 2021. PMID: 34174146 Review.
Cited by
-
Synthetic Protocols, Structural Activity Relationship, and Biological Activity of Piperazine and its Derivatives.Med Chem. 2024;20(8):753-780. doi: 10.2174/0115734064304396240415110015. Med Chem. 2024. PMID: 38685782 Review.
-
Building Up a Piperazine Ring from a Primary Amino Group via Catalytic Reductive Cyclization of Dioximes.Int J Mol Sci. 2023 Jul 22;24(14):11794. doi: 10.3390/ijms241411794. Int J Mol Sci. 2023. PMID: 37511552 Free PMC article.
-
One-pot four-component synthesis of novel isothiourea-ethylene-tethered-piperazine derivatives.RSC Adv. 2023 Nov 7;13(46):32772-32777. doi: 10.1039/d3ra06678a. eCollection 2023 Oct 31. RSC Adv. 2023. PMID: 37942451 Free PMC article.
-
Synthesis of novel antibacterial and antifungal dithiocarbamate-containing piperazine derivatives via re-engineering multicomponent approach.Heliyon. 2022 May 30;8(6):e09564. doi: 10.1016/j.heliyon.2022.e09564. eCollection 2022 Jun. Heliyon. 2022. PMID: 35669544 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

