DABCO bond cleavage for the synthesis of piperazine derivatives
- PMID: 35540608
- PMCID: PMC9075132
- DOI: 10.1039/c9ra07870c
DABCO bond cleavage for the synthesis of piperazine derivatives
Abstract
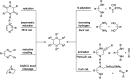
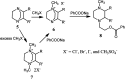
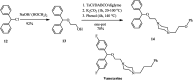
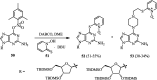
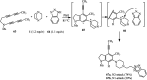
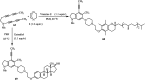
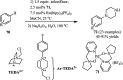
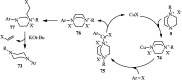
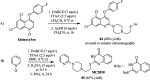
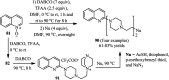
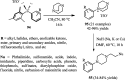
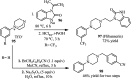
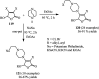
The applications of DABCO (1,4-diazabicyclo[2.2.2]octane) in the synthesis of piperazine derivatives including biologically active compounds via C-N bond cleavage are investigated in this review. Different reagents such as alkyl halides, aryl(heteroary) halides, carboxylic acids, diaryliodonium salts, tosyl halides, activated alkynes, benzynes etc. were applied for the preparation of the corresponding quaternary ammonium salts of DABCO, which are very good electrophiles for various nucleophiles such as phenols, thiophenols, thiols, alcohols, aliphatic and aromatic amines, sulfinates, phthalimide, indoles, NaN3, triazole and terazoles, NaCN, enols and enolates, halides, carboxylic acid salts etc. Besides preactivated DABCO salts, the in situ activation of DABCO in multicomponent reactions is also an efficient tactic in synthetic organic chemistry for the diversity oriented synthesis of drug-like piperazine derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


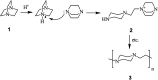



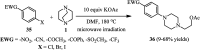

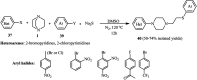

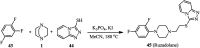
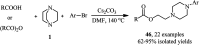

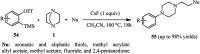

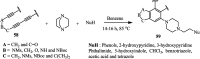
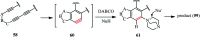





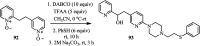
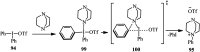

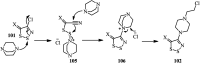
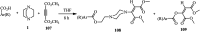
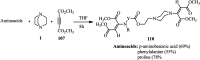



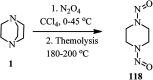



References
Publication types
LinkOut - more resources
Full Text Sources

