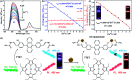
A stimuli responsive triplet-triplet annihilation upconversion system and its application as a ratiometric sensor for Fe3+ ions
- PMID: 35540611
- PMCID: PMC9074917
- DOI: 10.1039/c9ra06524e
A stimuli responsive triplet-triplet annihilation upconversion system and its application as a ratiometric sensor for Fe3+ ions
Abstract
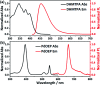
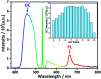
A ratiometric fluorescent sensor for the detection of Fe3+ ions is achieved based on triplet-triplet annihilation upconversion (TTA-UC) luminescence. A new anthracene derivative (named as DHTPA) is designed and synthesized and reveals similar optical properties to 9,10-diphenylanthracene (DPA) and is used as a stimuli responsive annihilator in a TTA-UC system due to its complexation ability. As a result, the UC emission can be significantly quenched by Fe3+ ions, while the phosphorescence (PL) emission of sensitizer palladium(ii) octaetylporphyrin (PdOEP) remains nearly constant, which makes the PL signal an appropriate internal reference for the UC signal. The UC and ratio signals (I UC/I PL) both reveal a good linear relationship with Fe3+ ion concentration, which for the first time makes the TTA-UC system a perfect ratiometric sensor for Fe3+ ion detection. This sensing method will open a novel avenue to achieve ratiometric sensors in chemical and biological fields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Logeman B. L. Thiele D. J. J. Biol. Chem. 2018;293:15497. doi: 10.1074/jbc.RA118.004802. - DOI - PMC - PubMed
- Lu Y. Z. Fu L. Li N. Ding J. Bai Y. N. Samaras P. Zeng R. J. Chemosphere. 2018;198:370. doi: 10.1074/jbc.RA118.004802. - DOI - PubMed
- Huang X. W. Boutroy É. Makvandi S. Beaudoin G. Corriveau L. Toni A. F. D. Miner. Deposita. 2019;54:525. doi: 10.1074/jbc.RA118.004802. - DOI
-
- Mun G. Jung S. H. Ahn A. Lee S. S. Choi M. Y. Kim D. H. Kim J. Y. Jung J. H. RSC Adv. 2016;6:53912. doi: 10.1039/C6RA09133D. - DOI
- Yazdanpanah A. Ghasimi D. S. M. Min G. K. Nakhla G. Hafez H. Keleman M. Environ. Sci. Pollut. Res. 2018;25:29240. doi: 10.1039/C6RA09133D. - DOI - PubMed
- Sharp P. A. Sugden B. Sambrook J. Biochemistry. 1973;12:3055. doi: 10.1039/C6RA09133D. - DOI - PubMed
- Tsubaki M. Srivastava R. B. Yu N. T. Biochemistry. 1982;21:1132. doi: 10.1039/C6RA09133D. - DOI - PubMed
- Outten F. W. Djaman O. G. Mol. Microbiol. 2010;52:861. doi: 10.1039/C6RA09133D. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

