One-step, high-yield synthesis of g-C3N4 nanosheets for enhanced visible light photocatalytic activity
- PMID: 35540641
- PMCID: PMC9076094
- DOI: 10.1039/c9ra08922e
One-step, high-yield synthesis of g-C3N4 nanosheets for enhanced visible light photocatalytic activity
Abstract
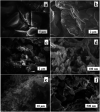
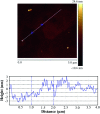
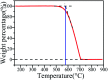
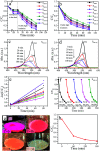
A facile template-free one-step synthesis method of ultrathin g-C3N4 nanosheets was developed through thermal polycondensation of melamine. The higher temperature, prolonged time and tightly sealed crucible reaction system contributed to the formation of ultrathin g-C3N4 nanosheets. The as-synthesized g-C3N4 nanosheets were applied to the visible light photocatalytic degradation of RhB. The photocatalytic activity was significantly enhanced with increased calcination temperature from 500 °C to 650 °C and prolonged calcination time from 4 h to 10 h. Interestingly, the obtained ultrathin g-C3N4 nanosheets simultaneously possess high yield and excellent photocatalytic activity. Moreover, g-C3N4 nanosheets can maintain photochemical stability after five consecutive runs. The remarkably enhanced photocatalytic activity can be interpreted as the synergistic effects of the enhanced crystallinity, the large surface area, the reduced layer thickness and size and the reduced number of defects. A new layer exfoliation and splitting mechanism of the formation of the ultrathin nanosheets was proposed. This work provides a new strategy to develop a facile eco-friendly template-free one-step synthesis method for potential large-scale synthesis of ultrathin nanosheets with high yield, high photocatalytic efficiency and stable activity for environmental and energetic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures









References
-
- Hoffmann M. R. Martin S. T. Choi W. Chem. Rev. 1995;95:69–96. doi: 10.1021/cr00033a004. - DOI
-
- Nie L. Zhang Q. Inorg. Chem. Front. 2017;4:1953–1962. doi: 10.1039/C7QI00651A. - DOI
-
- Song G. Chu Z. Jin W. Sun H. Chin. J. Chem. Eng. 2015;23:1326–1334. doi: 10.1016/j.cjche.2015.05.003. - DOI
-
- Khan M. A. Xia M. Mutahir S. Muhmood T. Catal. Sci. Technol. 2017;7:3017–3026. doi: 10.1039/C7CY00420F. - DOI
-
- Zhao J. Yan J. Jia H. Zhong S. Zhang X. Xu L. J. Mol. Catal. A: Chem. 2016;424:162–170. doi: 10.1016/j.molcata.2016.08.025. - DOI
LinkOut - more resources
Full Text Sources

