Sequential cycloaddition and ring expansion reaction of arynes and methylenebenzothiopheneones: synthesis of a benzo-fused eight-membered ring via sulfonium ylides
- PMID: 35540652
- PMCID: PMC9075993
- DOI: 10.1039/c9ra09332j
Sequential cycloaddition and ring expansion reaction of arynes and methylenebenzothiopheneones: synthesis of a benzo-fused eight-membered ring via sulfonium ylides
Abstract
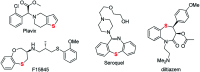
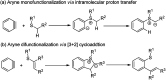
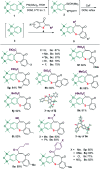
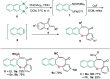
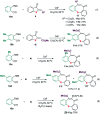
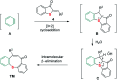
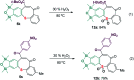
A sequential cycloaddition and ring expansion of arynes and methylenebenzothiopheneones has been disclosed. This strategy proceeds through a sulfonium ylides intermediate and allows for the efficient synthesis of a sulfur-containing benzo-fused eight-membered ring.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Please find the data reflecting the top 200 rankings from http://www.pharmacytimes.com/publications/issue/2012/July2012/Top-200-Dr..., 2012
-
-
For selected reviews on aryne chemistry, see:
- Takikawa H. Nishii A. Sakai T. Suzuki K. Chem. Soc. Rev. 2018;47:8030. doi: 10.1039/C8CS00350E. - DOI - PubMed
- Roy T. Biju A. T. Chem. Commun. 2018;54:2580. doi: 10.1039/C7CC09122B. - DOI - PubMed
- Shi J. Li Y. Li Y. Chem. Soc. Rev. 2017;46:1707. doi: 10.1039/C6CS00694A. - DOI - PubMed
- Yoshida S. Hosoya T. Chem. Lett. 2015;44:1450. doi: 10.1246/cl.150839. - DOI
- Pérez D. Peña D. Guitián E. Eur. J. Org. Chem. 2013:5981. doi: 10.1002/ejoc.201300470. - DOI
- Gampe C. M. Carreira E. M. Angew. Chem., Int. Ed. 2012;51:3766. doi: 10.1002/anie.201107485. - DOI - PubMed
- Tadross P. M. Stoltz B. M. Chem. Rev. 2012;112:3550. doi: 10.1021/cr200478h. - DOI - PubMed
- Bhunia A. Yetra S. R. Biju A. T. Chem. Soc. Rev. 2012;41:3140. doi: 10.1039/C2CS15310F. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

