Hexachlorobenzene exerts genotoxic effects in a humpback whale cell line under stable exposure conditions
- PMID: 35540658
- PMCID: PMC9076109
- DOI: 10.1039/c9ra05352b
Hexachlorobenzene exerts genotoxic effects in a humpback whale cell line under stable exposure conditions
Abstract
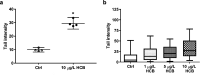
Humpback whales, like other polar wildlife, accumulate persistent organic pollutants. In Southern hemisphere populations, hexachlorobenzene (HCB) dominates the contaminant profiles. HCB is linked to a variety of health effects and is classified as a group 2B carcinogen, but the mechanism of action is a matter of contention. Potential toxicological effects to humpback whales remain entirely unknown. The recently established humpback whale fibroblast cell line (HuWa) offers an in vitro model for toxicological investigations. We here combine this novel cell line with a passive dosing strategy to investigate whale-specific toxicity of HCB. The relevant partitioning coefficients were determined to produce stable and predictable exposure concentrations in small-scale bioassays. The system was used to assess acute toxicity as well as genotoxicity of HCB to the HuWa cell line. While we found some transient reductions in metabolic activity, measured with the indicator dye alamarBlue, no clear acute toxic effects were discernible. Yet, a significant increase in DNA damage, detected in the alkaline comet assay, was found in HuWa cells exposed to 10 μg L-1 HCB during the sensitive phase of cell attachment. Collectively, this work provides a ready-to-use passive dosing system and delivers evidence that HCB elicits genotoxicity in humpback whale cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Linking pollutant exposure of humpback whales breeding in the Indian Ocean to their feeding habits and feeding areas off Antarctica.Environ Pollut. 2017 Jan;220(Pt B):1090-1099. doi: 10.1016/j.envpol.2016.11.032. Epub 2016 Nov 22. Environ Pollut. 2017. PMID: 27884466
-
A model to resolve organochlorine pharmacokinetics in migrating humpback whales.Environ Toxicol Chem. 2014 Jul;33(7):1638-49. doi: 10.1002/etc.2603. Epub 2014 Jun 3. Environ Toxicol Chem. 2014. PMID: 24733631
-
Establishment of the first humpback whale fibroblast cell lines and their application in chemical risk assessment.Aquat Toxicol. 2015 Oct;167:240-7. doi: 10.1016/j.aquatox.2015.08.005. Epub 2015 Aug 20. Aquat Toxicol. 2015. PMID: 26363275
-
Hexachlorobenzene as a persistent organic pollutant: Toxicity and molecular mechanism of action.Pharmacol Rep. 2017 Dec;69(6):1232-1239. doi: 10.1016/j.pharep.2017.06.013. Epub 2017 Jul 1. Pharmacol Rep. 2017. PMID: 29128804 Review.
-
Environmental toxicology and health effects associated with hexachlorobenzene exposure.Rev Environ Health. 2007 Jul-Sep;22(3):213-43. doi: 10.1515/reveh.2007.22.3.213. Rev Environ Health. 2007. PMID: 18078005 Review.
Cited by
-
Analysis of the Influence of Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus Strains on Changes in the Hexachlorobenzene Content in Fermented Mare Milk during Refrigerated Storage.Molecules. 2024 Jan 21;29(2):528. doi: 10.3390/molecules29020528. Molecules. 2024. PMID: 38276605 Free PMC article.
References
-
- Letcher R. J. Bustnes J. O. Dietz R. Jenssen B. M. Jørgensen E. H. Sonne C. Verreault J. Vijayan M. M. Gabrielsen G. W. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Total Environ. 2010;408:2995–3043. doi: 10.1016/j.scitotenv.2009.10.038. - DOI - PubMed
-
- Kallenborn R. Hung H. Brorström-Lundén E. Atmospheric long-range transport of persistent organic pollutants (POPs) into polar regions. Compr. Anal. Chem. 2015;67:411–432.
-
- Bengtson Nash S. M. Wild S. J. Hawker D. W. Cropp R. A. Hung H. Wania F. Xiao H. Bohlin-Nizzetto P. Bignert A. Broomhall S. Persistent organic pollutants in the East Antarctic atmosphere: inter-annual observations from 2010 to 2015 using high-flow-through passive sampling. Environ. Sci. Technol. 2017;51:13929–13937. doi: 10.1021/acs.est.7b04224. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

