NO reduction over an Al-embedded MoS2 monolayer: a first-principles study
- PMID: 35540686
- PMCID: PMC9075936
- DOI: 10.1039/c9ra05759e
NO reduction over an Al-embedded MoS2 monolayer: a first-principles study
Abstract
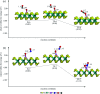
Converting toxic air pollutants such as nitric oxide (NO) and carbon monoxide (CO) into less harmful gases remains a critical challenge for many industrial technologies. Here, by performing first-principles calculations, we introduce a cheap, stable and novel catalyst for the conversion of NO and CO molecules into N2O and CO2 using Al-doped MoS2 (Al-MoS2). According to our results, dissociation of NO molecules on Al-MoS2 has a large energy barrier (3.62 eV), suggesting that it is impossible at ambient temperature. In contrast, the coadsorption of NO molecules to form (NO)2 moieties is characterized as the first step of the NO reduction process. The formed (NO)2 is unstable on Al-MoS2, and hence it is easily decomposed into N2O molecules, and an oxygen atom is adsorbed onto the Al atom (Oads). This reaction step is exothermic and needs an activation energy of 0.37 eV to be overcome. Next, the Oads moiety is removed from the Al atom by a CO molecule, and thereby the Al-MoS2 catalyst is recovered for the next round of reaction. The side reaction producing NO2 via the reaction of NO with the Oads moiety cannot proceed on Al-MoS2 due to its large activation energy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

