Ferrocene-functionalized polyheteroacenes for the use as cathode active material in rechargeable batteries
- PMID: 35540730
- PMCID: PMC9079884
- DOI: 10.1039/c8ra00129d
Ferrocene-functionalized polyheteroacenes for the use as cathode active material in rechargeable batteries
Abstract
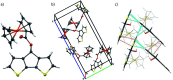
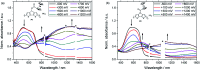
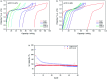
Herein we report the synthesis and characterization of new conjugated polymers bearing redox-active pendant groups for applications as cathode active materials in secondary batteries. The polymers comprise a ferrocene moiety immobilized at a poly(cyclopenta[2,1-b:3,4-b']dithiophene) (pCPDT, P1) or a poly(dithieno[3,2-b:2',3'-d]pyrrole) (pDTP, P2) backbone via an ester or an amide linker. Electrochemical and oxidative chemical polymerizations were performed in order to investigate the redox behaviour of the obtained polymers P1 and P2 and to synthesize materials on gram-scale for battery tests, respectively. During galvanostatic cycling in a typical battery environment, both polymers showed high reversible capacities of 90% and 87% of their theoretical capacity and excellent capacity retentions of 84% and 97% over 50 cycles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Choi J. Song H. Kim N. Kim F. S. Semicond. Sci. Technol. 2015;30:1–16.
- Guo X. Baumgarten M. Müllen K. Prog. Polym. Sci. 2013;38:1832–1908. doi: 10.1016/j.progpolymsci.2013.09.005. - DOI
-
- Novak P. Müller K. Santhanam K. S. V. Haas O. Chem. Rev. 1997;97:207–282. doi: 10.1021/cr941181o. - DOI - PubMed
- Song Z. Zhou H. Energy Environ. Sci. 2013;6:2280–2301. doi: 10.1039/C3EE40709H. - DOI
- Muench S. Wild A. Friebe C. Haupler B. Janoschka T. Schubert U. S. Chem. Rev. 2016;116:9438–9484. doi: 10.1021/acs.chemrev.6b00070. - DOI - PubMed
LinkOut - more resources
Full Text Sources

