Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1
- PMID: 35540737
- PMCID: PMC9079959
- DOI: 10.1039/c8ra02358a
Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1
Abstract
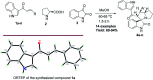
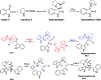
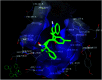
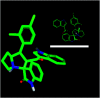
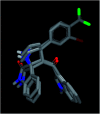
Spirooxindole is a promising chemo therapeutic agent. Possible targets include cancers of the liver, prostate, lung, stomach, colon, and breast. Here, we demonstrate a one-pot three-component reaction via a [3 + 2] cycloaddition/ring contraction sequence of a dipolarophile (activated alkene) with in situ-generated azomethine ylide (1,3-dipoles) without the use of any catalyst. The reaction provides efficient access to synthetically useful and biologically important spirooxindoles in high yield (69-94%) with high diastereoselectivity. The synthesized compounds were subjected to cytotoxicity evaluation using colorectal cancer (HCT-116), hepatocellular carcinoma (HepG2), and prostate cancer (PC-3) cells. Compounds 4i, 4j, and 4k showed potent cytotoxic activity and high selectivity against HCT-116 cells when compared to cisplatin. Meanwhile compound 4d retained high cytotoxic activity and selectivity against HepG2 and PC-3 cells in comparison to cisplatin. The mechanism of compound 4d was further studied using phosphodiesterase 1 enzyme and showed 74.2% inhibitory activity. A possible binding mode for compound 4d to PDE-1 was investigated by molecular modeling using OpenEye software. Pose predictions for the active compounds were demonstrated by ROCS alignments. Compound 4d has a special geometry and differs from other active compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Synthesis of new thiazolo-pyrrolidine-(spirooxindole) tethered to 3-acylindole as anticancer agents.Bioorg Chem. 2019 Feb;82:423-430. doi: 10.1016/j.bioorg.2018.10.036. Epub 2018 Oct 23. Bioorg Chem. 2019. PMID: 30508794
-
Novel spirooxindole-triazole derivatives: unveiling [3+2] cycloaddition reactivity through molecular electron density theory and investigating their potential cytotoxicity against HepG2 and MDA-MB-231 cell lines.Front Chem. 2024 Sep 30;12:1460384. doi: 10.3389/fchem.2024.1460384. eCollection 2024. Front Chem. 2024. PMID: 39403698 Free PMC article.
-
Stereoselective Synthesis of Spirooxindole Derivatives Using One-Pot Multicomponent Cycloaddition Reaction and Evaluation of Their Antiproliferative Efficacy.ACS Omega. 2020 Oct 16;5(42):27332-27343. doi: 10.1021/acsomega.0c03675. eCollection 2020 Oct 27. ACS Omega. 2020. PMID: 33134696 Free PMC article.
-
Chemo-/Regio-Selective Synthesis of Novel Functionalized Spiro[pyrrolidine-2,3'-oxindoles] under Microwave Irradiation and Their Anticancer Activity.Molecules. 2023 Sep 7;28(18):6503. doi: 10.3390/molecules28186503. Molecules. 2023. PMID: 37764279 Free PMC article.
-
Regio- and stereoselective synthesis of new spirooxindoles via 1,3-dipolar cycloaddition reaction: Anticancer and molecular docking studies.J Photochem Photobiol B. 2018 Mar;180:98-108. doi: 10.1016/j.jphotobiol.2018.01.026. Epub 2018 Jan 31. J Photochem Photobiol B. 2018. PMID: 29413708
Cited by
-
Synthesis of a New Class of Spirooxindole-Benzo[b]Thiophene-Based Molecules as Acetylcholinesterase Inhibitors.Molecules. 2020 Oct 13;25(20):4671. doi: 10.3390/molecules25204671. Molecules. 2020. PMID: 33066293 Free PMC article.
-
Construction of Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds as Acetylcholinesterase Inhibitors.ACS Omega. 2021 Nov 12;6(47):31539-31556. doi: 10.1021/acsomega.1c03978. eCollection 2021 Nov 30. ACS Omega. 2021. PMID: 34869980 Free PMC article.
-
Microwave-assisted protocol towards synthesis of heterocyclic molecules: a comparative analysis with conventional synthetic methodologies (years 2019-2023): a review.Mol Divers. 2025 Jun;29(3):2717-2763. doi: 10.1007/s11030-024-10981-y. Epub 2024 Sep 20. Mol Divers. 2025. PMID: 39302538 Review.
-
Three-Component [3+2] Cycloaddition for Regio- and Diastereoselective Synthesis of Spirooxindole-Pyrrolidines.New J Chem. 2022 Feb 28;46(8):3866-3870. doi: 10.1039/d1nj05538k. Epub 2022 Jan 20. New J Chem. 2022. PMID: 36157552 Free PMC article.
-
Indole Compounds in Oncology: Therapeutic Potential and Mechanistic Insights.Pharmaceuticals (Basel). 2024 Jul 10;17(7):922. doi: 10.3390/ph17070922. Pharmaceuticals (Basel). 2024. PMID: 39065774 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

