Enhanced desalination using a three-layer OTMS based superhydrophobic membrane for a membrane distillation process
- PMID: 35540818
- PMCID: PMC9078671
- DOI: 10.1039/c8ra01043a
Enhanced desalination using a three-layer OTMS based superhydrophobic membrane for a membrane distillation process
Abstract
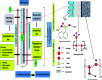
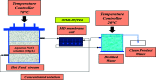
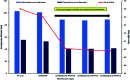
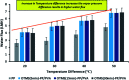
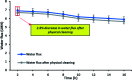
Superhydrophobic membranes are essential for improved seawater desalination. This study presents the successful casting of a three-layered membrane composed of a top superhydrophobic coating onto a polypropylene (PP) mat through simple sol-gel processing of octadecyltrimethoxysilane (OTMS), and the bottom layer was casted with hydrophilic poly(vinyl alcohol) (PVA) by using a knife casting technique; this membrane represents a novel class of improved-performance membranes consisting of a top superhydrophobic coating onto a hydrophobic PP mat and a hydrophilic layer (PVA) at the bottom. OTMSs are well known low-surface-energy materials that enhance superhydrophobicity, and they were observed to be the ideal chemical group for increasing the hydrophobicity of the PP mat. The PVA layer acted as base layer absorbing the condensed vapor and thus enhancing the vapor flux across the membrane. The hybrid three-layered membrane exhibited superhydrophobicity, with an average contact angle of more than 160°, and demonstrated high performance in terms of rejection and water flux. This study also examined the pore size distribution, surface roughness, surface area, tensile strength, water flux, and salt rejection of the fabricated membrane. The salt rejection level was calculated to be 99.7%, and a high permeate flux of approximately 6.7 LMH was maintained for 16 h.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Nguyen N. C. Chen S.-S. Nguyen H. T. Chen Y.-H. Ngo H. H. Guo W. Ray S. S. Chang H.-M. Le Q. H. Sep. Purif. Technol. 2017 doi: 10.1016/j.seppur.2017.01.011. - DOI
-
- Ray S. S. Chen S.-S. Li C.-W. Nguyen N. C. Nguyen H. T. RSC Adv. 2016;6:85495–85514. doi: 10.1039/C6RA14952A. - DOI
-
- Ray S. S. Chen S.-S. Hsu H.-T. Cao D.-T. Nguyen H.-T. Nguyen N. C. Sep. Purif. Technol. 2017;186:352–365. doi: 10.1016/j.seppur.2017.06.032. - DOI
-
- Curcio E. Drioli E. Sep. Purif. Rev. 2005;34:35–86. doi: 10.1081/SPM-200054951. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

