Creating tissue on chip constructs in microtitre plates for drug discovery
- PMID: 35540822
- PMCID: PMC9078682
- DOI: 10.1039/c8ra00849c
Creating tissue on chip constructs in microtitre plates for drug discovery
Abstract
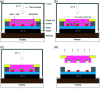
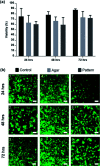
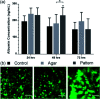
We report upon a novel coplanar dielectrophoresis (DEP) based cell patterning system for generating transferrable hepatic cell constructs, resembling a liver-lobule, in culture. The use of paper reinforced gel substrates provided sufficient strength to enable these constructs to be transfered into 96-well plates for long term functional studies, including in the future, drug development studies. Experimental results showed that hepatic cells formed DEP field-induced structures corresponding to an array of lobule-mimetic patterns. Hepatic viability was observed over a period of 3 days by the use of a fluorescent cell staining technique, whilst the liver specific functionality of albumin secretion showed a significant enhancement due to the layer patterning of cell lines (HepG2/C3A), compared to 2D patterned cells and un-patterned control. This "build and transfer" concept could, in future, also be adapted for the layer-by-layer construction of organs-on-chip in microtitre formats.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue.Lab Chip. 2013 Sep 21;13(18):3578-87. doi: 10.1039/c3lc50402f. Lab Chip. 2013. PMID: 23743812
-
Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap.Lab Chip. 2006 Jun;6(6):724-34. doi: 10.1039/b602036d. Epub 2006 May 3. Lab Chip. 2006. PMID: 16738722
-
nDEP-driven cell patterning and bottom-up construction of cell aggregates using a new bioelectronic chip.Acta Biomater. 2015 Apr;17:107-14. doi: 10.1016/j.actbio.2015.01.011. Epub 2015 Jan 14. Acta Biomater. 2015. PMID: 25595475
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
-
Next generation human skin constructs as advanced tools for drug development.Exp Biol Med (Maywood). 2017 Nov;242(17):1657-1668. doi: 10.1177/1535370217712690. Epub 2017 Jun 7. Exp Biol Med (Maywood). 2017. PMID: 28592171 Free PMC article. Review.
Cited by
-
Recent advances in liver-on-chips: Design, fabrication, and applications.Smart Med. 2023 Feb 12;2(1):e20220010. doi: 10.1002/SMMD.20220010. eCollection 2023 Feb. Smart Med. 2023. PMID: 39188562 Free PMC article. Review.
-
Liver microsystems in vitro for drug response.J Biomed Sci. 2019 Oct 28;26(1):88. doi: 10.1186/s12929-019-0575-0. J Biomed Sci. 2019. PMID: 31660980 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

