LC-ESI-MS/MS reveals the formation of reactive intermediates in brigatinib metabolism: elucidation of bioactivation pathways
- PMID: 35540908
- PMCID: PMC9077137
- DOI: 10.1039/c7ra10533a
LC-ESI-MS/MS reveals the formation of reactive intermediates in brigatinib metabolism: elucidation of bioactivation pathways
Abstract
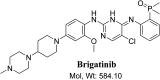
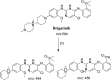
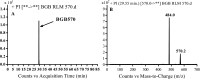
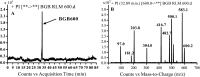
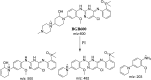
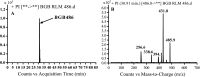
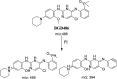
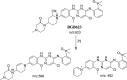
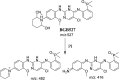
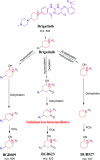
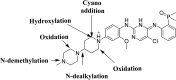
Brigatinib (BGB) is a newly approved anaplastic lymphoma kinase (ALK) inhibitor. On April 28, 2017, BGB was approved by the U.S. FDA for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. The toxicity profile of BGB includes nausea, fatigue, diarrhea, elevated lipase, dyspnoea, hypertension, hypoxia, pneumonia, elevated amylase, pulmonary embolism, elevated ALT, hyponatraemia and hypophosphatemia. Using LC-MS/MS, we investigated the in vitro phase I metabolism of for BGB in rat liver microsomes (RLMs). In the in vitro metabolism of BGB, iminium reactive intermediates were trapped by potassium cyanide forming a stable complex that can be characterized by LC-MS/MS. Four BGB in vitro phase I metabolites were identified. In vitro phase I metabolic pathways were N-dealkylation, α hydroxylation and α oxidation. Additionally, three iminium reactive metabolites were found and the bioactivation mechanisms were proposed. A piperidine ring was found to be responsible for BGB bioactivation. The presence of these three reactive metabolites may be the main reason for BGB side effects. A literature review showed no previous article reported the in vitro phase I metabolism study of BGB or structural identification of the formed reactive metabolites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

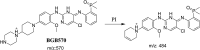

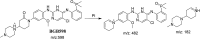

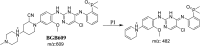


Similar articles
-
Identification of reactive intermediate formation and bioactivation pathways in Abemaciclib metabolism by LC-MS/MS: in vitro metabolic investigation.R Soc Open Sci. 2019 Jan 23;6(1):181714. doi: 10.1098/rsos.181714. eCollection 2019 Jan. R Soc Open Sci. 2019. PMID: 30800400 Free PMC article.
-
Characterization of reactive intermediates formation in dacomitinib metabolism and bioactivation pathways elucidation by LC-MS/MS: in vitro phase I metabolic investigation.RSC Adv. 2018 Nov 19;8(68):38733-38744. doi: 10.1039/c8ra06709k. eCollection 2018 Nov 16. RSC Adv. 2018. PMID: 35558335 Free PMC article.
-
Investigation of Fenebrutinib Metabolism and Bioactivation Using MS3 Methodology in Ion Trap LC/MS.Molecules. 2023 May 22;28(10):4225. doi: 10.3390/molecules28104225. Molecules. 2023. PMID: 37241965 Free PMC article.
-
Role of cyclic tertiary amine bioactivation to reactive iminium species: structure toxicity relationship.Curr Drug Metab. 2011 Jan;12(1):35-50. doi: 10.2174/138920011794520044. Curr Drug Metab. 2011. PMID: 21222587 Review.
-
Brigatinib: Novel ALK Inhibitor for Non-Small-Cell Lung Cancer.Ann Pharmacother. 2019 Jun;53(6):621-626. doi: 10.1177/1060028018824578. Epub 2019 Jan 13. Ann Pharmacother. 2019. PMID: 30638036 Review.
Cited by
-
Characterization of in vivo metabolites in rat urine following an oral dose of masitinib by liquid chromatography tandem mass spectrometry.Chem Cent J. 2018 May 15;12(1):61. doi: 10.1186/s13065-018-0429-y. Chem Cent J. 2018. PMID: 29766296 Free PMC article.
-
Sapitinib: reactive intermediates and bioactivation pathways characterized by LC-MS/MS.RSC Adv. 2019 Oct 16;9(57):32995-33006. doi: 10.1039/c9ra03926k. eCollection 2019 Oct 15. RSC Adv. 2019. PMID: 35529145 Free PMC article.
-
Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry.RSC Adv. 2022 Jul 21;12(32):20991-21003. doi: 10.1039/d2ra02848d. eCollection 2022 Jul 14. RSC Adv. 2022. PMID: 35919181 Free PMC article.
-
Paenibacillus polymyxa Antagonism towards Fusarium: Identification and Optimisation of Antibiotic Production.Toxins (Basel). 2023 Feb 9;15(2):138. doi: 10.3390/toxins15020138. Toxins (Basel). 2023. PMID: 36828452 Free PMC article.
-
Identification and characterization of in silico, in vivo, in vitro, and reactive metabolites of infigratinib using LC-ITMS: bioactivation pathway elucidation and in silico toxicity studies of its metabolites.RSC Adv. 2020 Apr 23;10(28):16231-16244. doi: 10.1039/c9ra10871h. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498820 Free PMC article.
References
-
- Ettinger D. S. Akerley W. Bepler G. Blum M. G. Chang A. Cheney R. T. Chirieac L. R. D'Amico T. A. Demmy T. L. Ganti A. K. P. J. Natl. Compr. Cancer Network. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

