Graphitic-phase carbon nitride-based electrochemiluminescence sensing analyses: recent advances and perspectives
- PMID: 35540965
- PMCID: PMC9080761
- DOI: 10.1039/c8ra02221f
Graphitic-phase carbon nitride-based electrochemiluminescence sensing analyses: recent advances and perspectives
Abstract
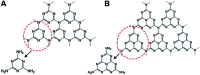
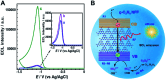
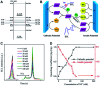
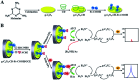
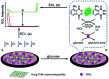
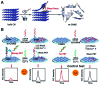
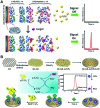
Graphitic-phase carbon nitride (g-C3N4) materials are important polymeric and metal-free semiconductors, and have attracted extensive attention as emerging electrochemiluminescence (ECL) emitters due to their wonderful optical and electronic properties. The g-C3N4-based ECL sensing analysis, as a research hotspot in analytical chemistry, offers an exquisite pathway to monitor target analytes with the advantages of low background signal, high sensitivity, desirable controllability, and simple instrumentation. Herein, we briefly describe the current research status of g-C3N4-based ECL assays along with versatile signaling strategies, introduce the preparation methods and ECL emission mechanisms of g-C3N4-dependent emitters, summarize their ECL sensing applications from 2012 to now, highlighting with special examples of metal ion and small molecule detection, nucleic acid bioanalysis, immunoassay, protein sensing, and cell-related determination. Finally, the prospects and challenges for future work are also explored to design more advanced ECL biosensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources

