On how ancillary ligand substitution affects the charge carrier dynamics in dye-sensitized solar cells
- PMID: 35540976
- PMCID: PMC9080644
- DOI: 10.1039/c8ra02968g
On how ancillary ligand substitution affects the charge carrier dynamics in dye-sensitized solar cells
Abstract
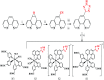
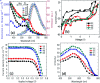
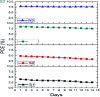
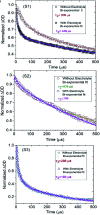
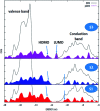
With respect to N3, a champion sensitizer in dye-sensitized solar cells (DSSCs), S3 which contained a phenTz (1,10-phenanthroline 5-tetrazole) ancillary ligand showed outstanding improvements in molar extinction coefficient (ε) from 10 681.8 to 12 954.5 M cm-1 as well as 0.92% and 0.9% increases in power conversion efficiency (PCE) and incident photon-to-electron conversion efficiency (IPCE), reaching 8.46% and 76.5%, respectively. To find the origin of the high performance of the DSSC based on a phenTz ancillary ligand, transient absorption spectroscopy (TA) was carried out and indicated that the rate of the regeneration reaction is about 100 times faster than the rate of recombination with the dye which is very exciting and surely a good reason to promote the phenTz ligand as a promising ancillary ligand in DSSCs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Green M. A. Hishikawa Y. Warta W. et al. . Prog. Photovoltaics. 2017;25:668.
-
- National Renewable Energy Laboratory Efficiency Chart http://www.nrel.gov/ncpv/images/efficiencychart.jpg, accessed January 2017
-
- Tiep H. Ku Z. Fan H. J. Adv. Energy Mater. 2016;6:1501420. doi: 10.1002/aenm.201501420. - DOI
LinkOut - more resources
Full Text Sources

