Poly(allylamine)/tripolyphosphate coacervates enable high loading and multiple-month release of weakly amphiphilic anionic drugs: an in vitro study with ibuprofen
- PMID: 35540986
- PMCID: PMC9080659
- DOI: 10.1039/c8ra02588f
Poly(allylamine)/tripolyphosphate coacervates enable high loading and multiple-month release of weakly amphiphilic anionic drugs: an in vitro study with ibuprofen
Abstract
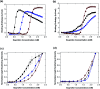
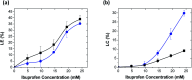
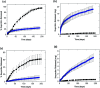
When synthetic polyamines, such poly(allylamine hydrochloride) (PAH), are mixed with crosslink-forming multivalent anions, they can undergo complex coacervation. This phenomenon has recently been exploited in various applications, ranging from inorganic material synthesis, to underwater adhesion, to multiple-month release of small, water-soluble molecules. Here, using ibuprofen as a model drug molecule, we show that these coacervates may be especially effective in the long-term release of weakly amphiphilic anionic drugs. Colloidal amphiphile/polyelectrolyte complex dispersions are first prepared by mixing the amphiphilic drug (ibuprofen) with PAH. Pentavalent tripolyphosphate (TPP) ions are then added to these dispersions to form ibuprofen-loaded PAH/TPP coacervates (where the strongly-binding TPP displaces the weaker-bound ibuprofen from the PAH amine groups). The initial ibuprofen/PAH binding leads to extremely high drug loading capacities (LC-values), where the ibuprofen comprises up to roughly 30% of the coacervate mass. Conversely, the dense ionic crosslinking of PAH by TPP results in very slow release rates, where the release of ibuprofen (a small, water-soluble drug) is extended over timescales that exceed 6 months. When ibuprofen is replaced with strong anionic amphiphiles, however (i.e., sodium dodecyl sulfate and sodium dodecylbenzenesulfonate), the stronger amphiphile/polyelectrolyte binding disrupts PAH/TPP association and sharply increases the coacervate solute permeability. These findings suggest that: (1) as sustained release vehicles, PAH/TPP coacervates might be very attractive for the encapsulation and multiple-month release of weakly amphiphilic anionic payloads; and (2) strong amphiphile incorporation could be useful for tailoring PAH/TPP coacervate properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Y. L. has a competing financial interest in a patent (US 9814778 B2) on the use PAH/TPP ionic networks in underwater adhesion and sustained release applications. The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Accelerating Payload Release from Complex Coacervates through Mechanical Stimulation.Polymers (Basel). 2023 Jan 23;15(3):586. doi: 10.3390/polym15030586. Polymers (Basel). 2023. PMID: 36771888 Free PMC article.
-
Ionically Cross-Linked Polymer Networks for the Multiple-Month Release of Small Molecules.ACS Appl Mater Interfaces. 2016 Feb;8(7):4323-35. doi: 10.1021/acsami.5b10070. Epub 2016 Feb 11. ACS Appl Mater Interfaces. 2016. PMID: 26811936 Free PMC article.
-
Highly Sustained Release of Bactericides from Complex Coacervates.ACS Appl Bio Mater. 2020 Dec 21;3(12):8427-8437. doi: 10.1021/acsabm.0c00763. Epub 2020 Nov 13. ACS Appl Bio Mater. 2020. PMID: 35019614
-
Ionically cross-linked poly(allylamine) as a stimulus-responsive underwater adhesive: ionic strength and pH effects.Langmuir. 2015 Feb 3;31(4):1564-74. doi: 10.1021/la504611x. Epub 2015 Jan 22. Langmuir. 2015. PMID: 25569307
-
Small Amphiphile-Based Coacervation.Chem Asian J. 2022 Dec 1;17(23):e202200938. doi: 10.1002/asia.202200938. Epub 2022 Oct 28. Chem Asian J. 2022. PMID: 36219462 Review.
Cited by
-
Polyelectrolyte-Surfactant Complex Nanofibrous Membranes for Antibacterial Applications.Polymers (Basel). 2024 Feb 1;16(3):414. doi: 10.3390/polym16030414. Polymers (Basel). 2024. PMID: 38337304 Free PMC article.
-
Accelerating Payload Release from Complex Coacervates through Mechanical Stimulation.Polymers (Basel). 2023 Jan 23;15(3):586. doi: 10.3390/polym15030586. Polymers (Basel). 2023. PMID: 36771888 Free PMC article.
-
Form Equals Function: Influence of Coacervate Architecture on Drug Delivery Applications.ACS Biomater Sci Eng. 2024 Nov 11;10(11):6766-6789. doi: 10.1021/acsbiomaterials.4c01105. Epub 2024 Oct 18. ACS Biomater Sci Eng. 2024. PMID: 39423330 Free PMC article. Review.
References
-
- Bungenberg de Jong H. G., in Colloid Science, ed. H. R. Kruyt, Elsevier, Amsterdam, 1949, ch. X, vol. II, pp. 335–432
-
- Bagaria H. G. Wong M. S. J. Mater. Chem. 2011;21:9454–9466. doi: 10.1039/C1JM10712G. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

