Honeycomb-patterned porous films fabricated via self-organization of Tb complex-loaded amphiphilic copolymers
- PMID: 35540989
- PMCID: PMC9080656
- DOI: 10.1039/c8ra02980f
Honeycomb-patterned porous films fabricated via self-organization of Tb complex-loaded amphiphilic copolymers
Abstract
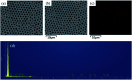
Amphiphilic copolymers, poly(styrene)-block-Tb complex (PS-b-Tb complex), were synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization. The honeycomb structured porous films were fabricated via dropping the PS-b-Tb complex copolymer solutions on glass substrates by the breath figures method (BFM). The structure and composition of the amphiphilic copolymer PS-b-Tb complex were confirmed by gel permeation chromatography (GPC), Fourier transform infrared spectroscopy (FT-IR) and 1H nuclear magnetic resonance spectroscopy (1H NMR). The surface morphology and elemental mapping of the highly ordered porous films were investigated by field emission scanning electron microscopy (FESEM), energy dispersive X-ray spectroscopy (EDX) and laser scanning confocal microscopy (LSCM). The results indicated that the solvent type and copolymer concentration can affect the surface morphology of the porous films. The average diameter of the pores in the porous films decreased with the polymer concentration and the molecular weight of the copolymers increased. The FESEM-EDX analysis further verified that the hydrophilic groups (Tb complex groups) were mainly distributed at the pore wall, instead of at the outer surface layer of the films, which was consistent with the LSCM results.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Fabrication of honeycomb-patterned porous films from PS-b-PNIPAM amphiphilic diblock copolymers synthesized via RITP.J Colloid Interface Sci. 2014 Apr 15;420:112-8. doi: 10.1016/j.jcis.2014.01.004. Epub 2014 Jan 13. J Colloid Interface Sci. 2014. PMID: 24559708
-
Honeycomb-structured films by multifunctional amphiphilic biodegradable copolymers: surface morphology control and biomedical application as scaffolds for cell growth.ACS Appl Mater Interfaces. 2011 Jul;3(7):2487-95. doi: 10.1021/am200371c. Epub 2011 Jul 6. ACS Appl Mater Interfaces. 2011. PMID: 21699231
-
Synthesis of carboxylic block copolymers via reversible addition fragmentation transfer polymerization for tooth erosion prevention.J Dent Res. 2014 Dec;93(12):1264-9. doi: 10.1177/0022034514551609. Epub 2014 Sep 23. J Dent Res. 2014. PMID: 25248611 Free PMC article.
-
Solution and Solid-State Behavior of Amphiphilic ABA Triblock Copolymers of Poly(acrylic acid-stat-styrene)-block-poly(butyl acrylate)-block-poly(acrylic acid-stat-styrene).Macromolecules. 2022 Nov 8;55(21):9726-9739. doi: 10.1021/acs.macromol.2c01299. Epub 2022 Oct 28. Macromolecules. 2022. PMID: 36397936 Free PMC article.
-
Brush-type amphiphilic diblock copolymers from "living"/controlled radical polymerizations and their aggregation behavior.Langmuir. 2005 Aug 2;21(16):7180-5. doi: 10.1021/la051038y. Langmuir. 2005. PMID: 16042439
References
-
- He Y. Chen Y. Xu Q. Xu J. Weng J. ACS Appl. Mater. Interfaces. 2017;9:7826–7833. - PubMed
-
- Bera S. Pal M. Sarkar S. Jana S. ACS Appl. Mater. Interfaces. 2017;9:4420–4424. - PubMed
-
- Zhang J. Meng Z. Liu J. Schlaich C. Yu Z. Deng X. J. Mater. Chem. A. 2017;5:16369–16375.
-
- Yao B. Zhu Q. Yao L. Hao J. Appl. Surf. Sci. 2015;332:287–294. doi: 10.1016/j.apsusc.2015.01.170. - DOI
LinkOut - more resources
Full Text Sources

