Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system
- PMID: 35540999
- PMCID: PMC9080684
- DOI: 10.1039/c8ra01467a
Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system
Abstract
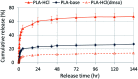
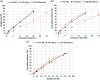
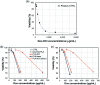
The drug release profiles of doxorubicin-loaded electrospun fiber mats were investigated with regard to drug-polymer miscibility, fiber wettability and degradability. Doxorubicin in hydrophilic form (Dox-HCl) and hydrophobic free base form (Dox-base) was employed as model drugs, and an aliphatic polyester, poly(lactic acid) (PLA), was used as a drug-carrier matrix. When hydrophilic Dox-HCl was directly mixed with PLA solution, drug molecules formed large aggregates on the fiber surface or in the fiber core, due to poor drug-polymer compatibility. Drug aggregates on the fiber surface contributed to the rapid initial release. The hydrophobic form of Dox-base was dispersed better with PLA matrix compared to Dox-HCl. When dimethyl sulfoxide (DMSO) was used as the solvent for Dox-HCl, the miscibility of drug in the polymer matrix was significantly improved, forming a quasi-monolithic solution scheme. The drug release from this monolithic matrix was slowest, and this slow release led to a lower toxicity to hepatocellular carcinoma. When an enzyme was used to promote PLA degradation, the release rates were closely correlated with degradation rates, demonstrating degradation was the dominant release mechanism. The possible drug release mechanisms were speculated based on the release kinetics. The results suggest that manipulation of drug-polymer miscibility and polymer degradability can be an effective means of designing drug release profiles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Doxorubicin Release Controlled by Induced Phase Separation and Use of a Co-Solvent.Materials (Basel). 2018 Apr 26;11(5):681. doi: 10.3390/ma11050681. Materials (Basel). 2018. PMID: 29701714 Free PMC article.
-
Polylactide-graft-doxorubicin nanoparticles with precisely controlled drug loading for pH-triggered drug delivery.Biomacromolecules. 2014 Feb 10;15(2):524-32. doi: 10.1021/bm401471p. Epub 2014 Jan 29. Biomacromolecules. 2014. PMID: 24446700
-
Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery.Biomaterials. 2009 Jun;30(16):3009-19. doi: 10.1016/j.biomaterials.2009.02.011. Epub 2009 Feb 27. Biomaterials. 2009. PMID: 19250665
-
Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy.Int J Nanomedicine. 2019 Jul 5;14:4961-4974. doi: 10.2147/IJN.S209325. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31308666 Free PMC article.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Preparation and in vitro release kinetics of nitrendipine-loaded PLLA-PEG-PLLA microparticles by supercritical solution impregnation process.RSC Adv. 2019 May 23;9(28):16167-16175. doi: 10.1039/c9ra01068h. eCollection 2019 May 20. RSC Adv. 2019. PMID: 35521402 Free PMC article.
-
Berberine-loaded polylactic acid nanofiber scaffold as a drug delivery system: The relationship between chemical characteristics, drug-release behavior, and antibacterial efficiency.Beilstein J Nanotechnol. 2024 Jan 12;15:71-82. doi: 10.3762/bjnano.15.7. eCollection 2024. Beilstein J Nanotechnol. 2024. PMID: 38229677 Free PMC article.
-
Encapsulation of Pharmaceutical and Nutraceutical Active Ingredients Using Electrospinning Processes.Nanomaterials (Basel). 2021 Jul 30;11(8):1968. doi: 10.3390/nano11081968. Nanomaterials (Basel). 2021. PMID: 34443799 Free PMC article. Review.
-
One-Pot Synthesis and Characterization of Magnetic α-Fe2O3/CuO/CuFe2O4 Nanocomposite for Multifunctional Therapeutic Applications.ChemistryOpen. 2025 Feb;14(2):e202400277. doi: 10.1002/open.202400277. Epub 2024 Oct 30. ChemistryOpen. 2025. PMID: 39473328 Free PMC article.
-
Water-Resistant Mechanoluminescent Electrospun Fabrics with Protected Sensitivity in Wet Condition via Plasma-Enhanced Chemical Vapor Deposition Process.Polymers (Basel). 2020 Jul 31;12(8):1720. doi: 10.3390/polym12081720. Polymers (Basel). 2020. PMID: 32751871 Free PMC article.
References
LinkOut - more resources
Full Text Sources

