Rapid quantitation of multiple ions released from HeLa cells during emodin induced apoptosis by low-cost capillary electrophoresis with capacitively coupled contactless conductivity detection
- PMID: 35541120
- PMCID: PMC9080565
- DOI: 10.1039/c8ra00492g
Rapid quantitation of multiple ions released from HeLa cells during emodin induced apoptosis by low-cost capillary electrophoresis with capacitively coupled contactless conductivity detection
Abstract
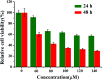
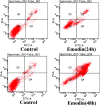
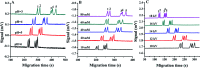
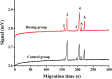
Change in cation concentration, including that of potassium and sodium, is characteristic of apoptosis, therefore it is significant to detect cation concentration changes. In this work a rapid, sensitive, and practical method was developed for the determination of Na+ and K+ concentration in HeLa cells during emodin induced apoptosis by a low-cost capillary electrophoresis device with capacitively coupled contactless conductivity detection (CE-C4D). Under the optimized conditions, both ions were baseline separated in 4 min with 40 mM MES/40 mM His containing 1 mM 18-crown-6 as the separation buffer at pH 6.0. The limit of detections (LODs) and limit of quantifications (LOQs) were 0.47-1.15 μM and 1.58-3.86 μM, respectively. The precision for migration times and peak areas was below 0.56% and 3.74%, respectively. The data proved that the concentration of cations in cells can be accurately quantified. It was found that the K+ concentration decreased from 82.2 μM to 52.7 μM, and the Na+ concentration increased from 62.4 μM to 127.2 μM during the process of apoptosis when the cell density was 1 × 105 cells per mL. The low-cost CE-C4D provides a convenient way to decipher the interaction of Na+ and K+ in the regulation of cell apoptosis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
[Multimaterial 3D-printed contactless conductivity/laser-induced fluorescence dual-detection cell for capillary electrophoresis].Se Pu. 2021 Aug;39(8):921-926. doi: 10.3724/SP.J.1123.2021.02021. Se Pu. 2021. PMID: 34212593 Free PMC article. Chinese.
-
A compact and high-performance setup of capillary electrophoresis with capacitively coupled contactless conductivity detection (CE-C4D).Analyst. 2024 May 13;149(10):3034-3040. doi: 10.1039/d4an00354c. Analyst. 2024. PMID: 38624147
-
A Highly Portable Smartphone-Based Capillary Electrophoresis with Capacitively Coupled Contactless Conductivity Detection.Sensors (Basel). 2025 Apr 4;25(7):2303. doi: 10.3390/s25072303. Sensors (Basel). 2025. PMID: 40218815 Free PMC article.
-
Application of Capillary Electrophoresis with Capacitively Coupled Contactless Conductivity Detection (CE-C4D): 2017-2020.Crit Rev Anal Chem. 2022;52(3):535-543. doi: 10.1080/10408347.2020.1809340. Epub 2020 Aug 24. Crit Rev Anal Chem. 2022. PMID: 32835492 Review.
-
Application of capillary electrophoresis with capacitively coupled contactless conductivity detection (CE-C4 D): An update.Biomed Chromatogr. 2017 Sep;31(9). doi: 10.1002/bmc.3945. Epub 2017 Feb 27. Biomed Chromatogr. 2017. PMID: 28178368 Review.
Cited by
-
Spatial Distribution of Intracellular Ion Concentrations in Aggregate-Forming HeLa Cells Analyzed by μ-XRF Imaging.ChemistryOpen. 2022 Apr;11(4):e202200024. doi: 10.1002/open.202200024. ChemistryOpen. 2022. PMID: 35363437 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

