Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene
- PMID: 35541305
- PMCID: PMC9080002
- DOI: 10.1039/c8ra01783b
Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene
Erratum in
-
Correction: Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene.RSC Adv. 2019 Mar 18;9(16):8934. doi: 10.1039/c9ra90021g. eCollection 2019 Mar 15. RSC Adv. 2019. PMID: 35532502 Free PMC article.
Abstract
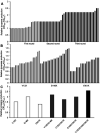
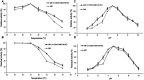
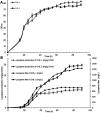
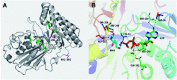
Lycopene is a terpenoid pigment that has diverse applications in the fields of food and medicine. Metabolic engineering in microbial hosts has shown that mevalonate kinase (MK, EC2.7.1.366) is one of the rate-limiting enzymes in the lycopene synthetic pathway. In this study, a directed evolution strategy in Escherichia coli was used to optimize the activity of Saccharomyces cerevisiae MK. Using three rounds of error-prone PCR; screening the development of a lycopene-dependent color reaction; and combinatorial site-specific saturation mutagenesis, three activity-enhancing mutations were identified: V13D, S148I, and V301E. V13D was near the MK catalytic center, in the β-sheet that forms a salt-bridge with nearby Arg-248. S148I was located in the α-helix lid and improved the stability of the α-helix. V301E may increase MK folding by influencing its secondary structure. The K m (RS)-mevalonate of purified mutant MK decreased by 74% compared with the K m (RS)-mevalonate of the wild-type MK, and the K cat (RS)-mevalonate was improved by 26% compared with wild type. Fermentation experiments revealed that lycopene production of the mutant MK increased 2.4-fold compared with wild-type MK.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures
Similar articles
-
Enhancement of the catalytic activity of Isopentenyl diphosphate isomerase (IDI) from Saccharomyces cerevisiae through random and site-directed mutagenesis.Microb Cell Fact. 2018 Apr 30;17(1):65. doi: 10.1186/s12934-018-0913-z. Microb Cell Fact. 2018. PMID: 29712558 Free PMC article.
-
Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering.Metab Eng. 2015 Jul;30:69-78. doi: 10.1016/j.ymben.2015.04.009. Epub 2015 May 8. Metab Eng. 2015. PMID: 25959020
-
Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering.Microb Cell Fact. 2016 Jun 21;15(1):113. doi: 10.1186/s12934-016-0509-4. Microb Cell Fact. 2016. PMID: 27329233 Free PMC article.
-
Polishing the craft of genetic diversity creation in directed evolution.Biotechnol Adv. 2013 Dec;31(8):1707-21. doi: 10.1016/j.biotechadv.2013.08.021. Epub 2013 Sep 6. Biotechnol Adv. 2013. PMID: 24012599 Review.
-
Metabolic Engineering Escherichia coli for the Production of Lycopene.Molecules. 2020 Jul 9;25(14):3136. doi: 10.3390/molecules25143136. Molecules. 2020. PMID: 32659911 Free PMC article. Review.
Cited by
-
Characterization of novel mevalonate kinases from the tardigrade Ramazzottius varieornatus and the psychrophilic archaeon Methanococcoides burtonii.Acta Crystallogr D Struct Biol. 2024 Mar 1;80(Pt 3):203-215. doi: 10.1107/S2059798324001360. Epub 2024 Feb 27. Acta Crystallogr D Struct Biol. 2024. PMID: 38411551 Free PMC article.
-
De novo biosynthesis of τ-cadinol in engineered Escherichia coli.Bioresour Bioprocess. 2022 Mar 21;9(1):29. doi: 10.1186/s40643-022-00521-7. Bioresour Bioprocess. 2022. PMID: 38647768 Free PMC article.
-
Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli.Nat Prod Rep. 2022 Jan 26;39(1):90-118. doi: 10.1039/d1np00025j. Nat Prod Rep. 2022. PMID: 34231643 Free PMC article. Review.
-
Enhancing limonene production by probing the metabolic network through time-series metabolomics data.Metabolomics. 2025 May 7;21(3):61. doi: 10.1007/s11306-025-02254-y. Metabolomics. 2025. PMID: 40335836
-
Functional diversity and metabolic engineering of plant-specialized metabolites.Life Metab. 2022 Aug 25;1(2):109-121. doi: 10.1093/lifemeta/loac019. eCollection 2022 Oct. Life Metab. 2022. PMID: 39872355 Free PMC article. Review.
References
-
- Rao A. V. Agarwal S. Nutr. Res. 1999;19:305–323. doi: 10.1016/S0271-5317(98)00193-6. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

