Self-radiolysis of tritiated water. 4. The scavenging effect of azide ions (N3 -) on the molecular hydrogen yield in the radiolysis of water by 60Co γ-rays and tritium β-particles at room temperature
- PMID: 35541471
- PMCID: PMC9077374
- DOI: 10.1039/c7ra12397c
Self-radiolysis of tritiated water. 4. The scavenging effect of azide ions (N3 -) on the molecular hydrogen yield in the radiolysis of water by 60Co γ-rays and tritium β-particles at room temperature
Abstract
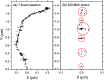
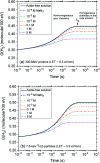
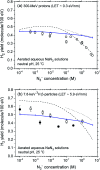
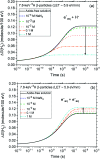
The effect of the azide ion N3 - on the yield of molecular hydrogen in water irradiated with 60Co γ-rays (∼1 MeV Compton electrons) and tritium β-electrons (mean electron energy of ∼7.8 keV) at 25 °C is investigated using Monte Carlo track chemistry simulations in conjunction with available experimental data. N3 - is shown to interfere with the formation of H2 through its high reactivity towards hydrogen atoms and, but to a lesser extent, hydrated electrons, the two major radiolytic precursors of the H2 yield in the diffusing radiation tracks. Chemical changes are observed in the H2 scavengeability depending on the particular type of radiation considered. These changes can readily be explained on the basis of differences in the initial spatial distribution of primary radiolytic species (i.e., the structure of the electron tracks). In the "short-track" geometry of the higher "linear energy transfer" (LET) tritium β-electrons (mean LET ∼5.9 eV nm-1), radicals are formed locally in much higher initial concentration than in the isolated "spurs" of the energetic Compton electrons (LET ∼0.3 eV nm-1) generated by the cobalt-60 γ-rays. As a result, the short-track geometry favors radical-radical reactions involving hydrated electrons and hydrogen atoms, leading to a clear increase in the yield of H2 for tritium β-electrons compared to 60Co γ-rays. These changes in the scavengeability of H2 in passing from tritium β-radiolysis to γ-radiolysis are in good agreement with experimental data, lending strong support to the picture of tritium β-radiolysis mainly driven by the chemical action of short tracks of high local LET. At high N3 - concentrations (>1 M), our H2 yield results for 60Co γ-radiolysis are also consistent with previous Monte Carlo simulations that suggested the necessity of including the capture of the precursors to the hydrated electrons (i.e., the short-lived "dry" electrons prior to hydration) by N3 -. These processes tend to reduce significantly the yields of H2, as is observed experimentally. However, this dry electron scavenging at high azide concentrations is not seen in the higher-LET 3H β-radiolysis, leading us to conclude that the increased amount of intra-track chemistry intervening at early time under these conditions favors the recombination of these electrons with their parent water cations at the expense of their scavenging by N3 -.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
References
-
- von Sonntag C., Free-Radical-Induced DNA Damage and Its Repair, Springer-Verlag, Berlin, 2006
-
- McCracken D. R., Tsang K. T. and Laughton P. J., Aspects of the physics and chemistry of water radiolysis by fast neutrons and fast electrons in nuclear reactors, Report AECL No. 11895, Atomic Energy of Canada Limited, Chalk River, Ontario, Canada, 2009
-
- Spinks J. W. T. and Woods R. J., An Introduction to Radiation Chemistry, Wiley, New York, 3rd edn, 1990
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

