2'-Fluoro-c-di-GMP as an oral vaccine adjuvant
- PMID: 35541605
- PMCID: PMC9076492
- DOI: 10.1039/c9ra08310c
2'-Fluoro-c-di-GMP as an oral vaccine adjuvant
Abstract
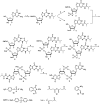
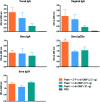
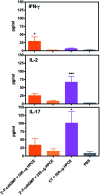
Bis-(3'-5')-cyclic dimeric 2'-deoxy-2'-fluoroguanosine monophosphate (2'-F-c-di-GMP) was synthesized through the modified H-phosphonate chemistry. Oral immunization of C57BL/6 mice with Helicobacter pylori cell-free sonicate extract adjuvanted with 2'-F-c-di-GMP led to the production of antigen-specific antibodies in feces and sera, and lowered bacterial counts in the stomach upon post-vaccination infections in immunized mice. Similarly, oral vaccination of BALB/c mice with flagillin proteins from Clostridium difficile and Listeria monocytogenes adjuvanted with 2'-F-c-di-GMP led to production of antigen-specific antibodies both systemically and mucosally. The adjuvanticity of 2'-F-c-di-GMP is associated with the enhanced induction of interferon γ. These results demonstrated the excellent oral adjuvanticity of 2'-F-c-di-GMP.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Work described in this manuscript is partially disclosed in WO patent 2015/074145A1 and US patent 10092644.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

