Facile one-pot synthesis of novel N-benzimidazolyl-α-arylnitrones catalyzed by salts of transition metals
- PMID: 35541607
- PMCID: PMC9076553
- DOI: 10.1039/c9ra08570j
Facile one-pot synthesis of novel N-benzimidazolyl-α-arylnitrones catalyzed by salts of transition metals
Abstract
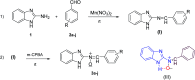
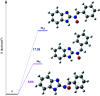
A novel series of N-benzimidazol-2-yl-α-aryl nitrones 3a-j is synthesized via simple one-pot condensation/oxidation of 2-aminobenzimidazole, an aromatic aldehyde and m-chloro perbenzoic acid (m-CPBA) as an effective oxidant using Mn(NO3)2·6H2O as an efficient catalyst at room temperature. All synthesized N-benzimidazolyl nitrones were identified using FTIR, NMR and mass spectroscopy. Also, stability energy theory calculations were performed and 1H NMR computational spectra were generated for the isomeric structures of 3a; the results show that the stability order is oxaziridine (4) followed by the nitrones 3a E and 3a Z . Also, comparing the computational spectroscopy results with the experimental data shows great accordance with nitrone 3a E . Among the remarkable points of this protocol, stable N-heterocyclic nitrones were prepared for the first time from raw materials under mild oxidative conditions. Therefore, they can easily be applied as high-potential intermediates for synthesizing valuable heterocycles in mild conditions. Due to benefits such as the use of inexpensive and available catalysts, short reaction times, high yields, facile workup to obtain pure product, and facile separation of the side product (m-chlorobenzoic acid), this simple protocol complies greatly with the principles of green chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


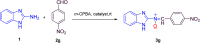
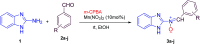

References
-
- Padwa A. and Pearson W. H., in Synthetic application of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products, John Wiley and Sons, Hoboken/NJ, vol. 59, 2003, ISBN: 978-0-471-28061-3
-
- Smith L. Chem. Rev. 1938;23:193–285. doi: 10.1021/cr60075a001. - DOI
-
- Hamer J. M. Macaluso A. Chem. Rev. 1964;64:473–495. doi: 10.1021/cr60230a006. - DOI
-
- Zhu T. Xiang J. Liu Z. Dang Q. Bai X. Synlett. 2015;26:238–242. doi: 10.1055/s-0034-1380120. - DOI
LinkOut - more resources
Full Text Sources

