Time-dependent and Pb-dependent antagonism and synergism towards Vibrio qinghaiensis sp.-Q67 within heavy metal mixtures
- PMID: 35541923
- PMCID: PMC9082770
- DOI: 10.1039/c8ra04191a
Time-dependent and Pb-dependent antagonism and synergism towards Vibrio qinghaiensis sp.-Q67 within heavy metal mixtures
Abstract
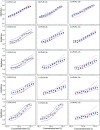
Toxicity interaction has aroused many researchers' interest in the combined toxicity of pollutants. Recently, some published studies have shown that the toxicity of some mixture pollutants is time dependent and well correlated with certain components in the mixture. Therefore, to investigate whether toxicity interaction is affected by the exposure time or certain components, synergism and antagonism within typical environmental contaminants of heavy metal mixtures were analyzed in different exposure times. Herein, three binary and one ternary mixture systems were designed by using three heavy metals: cadmium chloride, lead chloride (Pb) and manganese(ii) chloride tetrahydrate (Mn). For each mixture system, five mixture rays with different concentration ratios were arranged by direct equipartition ray design and uniform design ray methods. The toxicities of the three heavy metals and 20 mixture rays towards photobacteria Vibrio qinghaiensis sp.-Q67 (Q67) were determined by the established time-dependent microplate toxicity analysis (t-MTA) in different exposure durations of 0.25, 2, 4, 8 and 12 h. It was shown that the toxicities of three heavy metals (Cd, Pb and Mn) as well as their binary and ternary mixture rays to Q67 were also time dependent, but different metals or mixture rays showed different time characteristics. Surprisingly, some mixture rays exhibited antagonism or synergism with time dependency and the time characteristics varied in different mixture systems. Furthermore, the binary or ternary mixture systems with Pb displayed antagonism, while the Cd-Mn mixture system without Pb exhibited additive action or synergism, which indicated that Pb was probably the causative agent of antagonism produced by mixtures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Boltes K. and González-Naranjo V., Ecological Risk Assessment of Ibuprofen in Aquatic Environments: An Approach for Complex Mixture of Contaminants, Nova Publishers, 2013, pp. 85–158
LinkOut - more resources
Full Text Sources

