MoS2 formation induced by amorphous MoS3 species under lubricated friction
- PMID: 35541938
- PMCID: PMC9082756
- DOI: 10.1039/c8ra03317j
MoS2 formation induced by amorphous MoS3 species under lubricated friction
Abstract
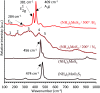
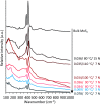
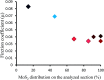
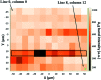
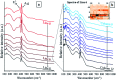
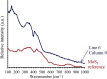
Amide molybdate has been recently introduced as a friction modifier for tribological applications. Combined with zinc dithiophosphate (ZDDP) and fatty amines, it provides an ultralow friction coefficient. The ultimate product of Mo compound transformations in tribological contact, due to frictional heating and shearing, as well as chemical interactions with oil additives, is molybdenum sulfide (MoS2). Understanding the decomposition of amide molybdate leading to MoS2 is of primary importance to the optimization of the design of lubricant formulations. This study focuses on the investigation by Raman spectroscopy of amide molybdate decomposition intermediates. Raman spectra of tribofilms, obtained after friction tests under different temperatures and pressures, revealed the formation of an amorphous MoS3 intermediate coexisting with MoS2. However, under severe conditions, the tribofilms are mostly composed of MoS2.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources

