P-Stereodefined phosphorothioate analogs of glycol nucleic acids-synthesis and structural properties
- PMID: 35542141
- PMCID: PMC9082371
- DOI: 10.1039/c8ra05568h
P-Stereodefined phosphorothioate analogs of glycol nucleic acids-synthesis and structural properties
Abstract
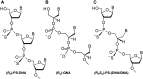
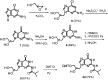
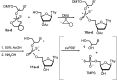
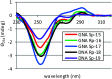
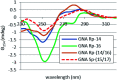
Enantiomerically pure, protected acyclic nucleosides of the GNA type (glycol nucleic acids) (GN'), obtained from (R)-(+)- and (S)-(-)-glycidols and the four canonical DNA nucleobases (Ade, Cyt, Gua and Thy), were transformed into 3'-O-DMT-protected 2-thio-4,4-pentamethylene-1,3,2-oxathiaphospholane derivatives (OTP-GN') containing a second stereogenic center at the phosphorus atom. These monomers were chromatographically separated into P-diastereoisomers, which were further used for the synthesis of P-stereodefined "dinucleoside" phosphorothioates GNPST (GN = GA, GC, GG, GT). The absolute configuration at the phosphorus atom for all eight GNPST was established enzymatically and verified chemically, and correlated with chromatographic mobility of the OTP-GN' monomers on silica gel. The GNPS units (derived from (R)-(+)-glycidol) were introduced into self-complementary PS-(DNA/GNA) octamers of preselected, uniform absolute configuration at P-atoms. Thermal dissociation experiments showed that the thermodynamic stability of the duplexes depends on the stereochemistry of the phosphorus centers and relative arrangement of the GN units in the oligonucleotide strands. These results correlate with the changes of conformation assessed from circular dichroism spectra.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

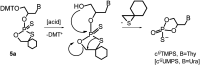


Similar articles
-
Synthesis and hybridizing properties of P-stereodefined chimeric [PS]-{DNA:RNA} and [PS]-{DNA:(2'-OMe)-RNA} oligomers.RSC Adv. 2022 Sep 21;12(41):26815-26824. doi: 10.1039/d2ra04855h. eCollection 2022 Sep 16. RSC Adv. 2022. PMID: 36320848 Free PMC article.
-
Homopurine RP-stereodefined phosphorothioate analogs of DNA with hampered Watson-Crick base pairings form Hoogsteen paired parallel duplexes with (2'-OMe)-RNAs.Org Biomol Chem. 2019 May 8;17(18):4611-4620. doi: 10.1039/c8ob03112f. Org Biomol Chem. 2019. PMID: 31017142
-
Thermal stability and conformation of antiparallel duplexes formed by P-stereodefined phosphorothioate DNA/LNA chimeric oligomers with DNA and RNA matrices.Org Biomol Chem. 2015 Oct 21;13(39):10032-40. doi: 10.1039/c5ob01474c. Org Biomol Chem. 2015. PMID: 26293357
-
Synthesis of phosphorothioate oligonucleotides with stereodefined phosphorothioate linkages.Curr Protoc Nucleic Acid Chem. 2003 Oct;Chapter 4:Unit 4.17. doi: 10.1002/0471142700.nc0417s14. Curr Protoc Nucleic Acid Chem. 2003. PMID: 18428907 Review.
-
Synthesis and chemical transformations of glycol nucleic acid (GNA) nucleosides.Bioorg Chem. 2023 Dec;141:106921. doi: 10.1016/j.bioorg.2023.106921. Epub 2023 Oct 17. Bioorg Chem. 2023. PMID: 37871392 Review.
Cited by
-
Artificial genetic polymers against human pathologies.Biol Direct. 2022 Dec 6;17(1):39. doi: 10.1186/s13062-022-00353-7. Biol Direct. 2022. PMID: 36474260 Free PMC article. Review.
-
Asymmetric Synthesis of Stereogenic Phosphorus P(V) Centers Using Chiral Nucleophilic Catalysis.Molecules. 2021 Jun 15;26(12):3661. doi: 10.3390/molecules26123661. Molecules. 2021. PMID: 34203996 Free PMC article.
-
An efficient alternative to DBU in the oxathiaphospholane (OTP) method for the solid phase synthesis of P-stereodefined phosphorothioate analogs.RSC Adv. 2024 Jul 4;14(29):21174-21179. doi: 10.1039/d4ra02833c. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38966816 Free PMC article.
-
Synthesis and hybridizing properties of P-stereodefined chimeric [PS]-{DNA:RNA} and [PS]-{DNA:(2'-OMe)-RNA} oligomers.RSC Adv. 2022 Sep 21;12(41):26815-26824. doi: 10.1039/d2ra04855h. eCollection 2022 Sep 16. RSC Adv. 2022. PMID: 36320848 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

