Global changes in gene expression related to Opisthorchis felineus liver fluke infection reveal temporal heterogeneity of a mammalian host response
- PMID: 35542180
- PMCID: PMC9079687
- DOI: 10.1016/j.fawpar.2022.e00159
Global changes in gene expression related to Opisthorchis felineus liver fluke infection reveal temporal heterogeneity of a mammalian host response
Abstract
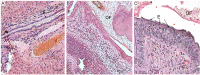
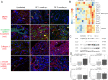
The food-borne trematode Opisthorchis felineus colonizes bile ducts of the liver of fish-eating mammals including humans. Among chronically infected individuals, this opisthorchiasis involves hepatobiliary problems, including chronic inflammation, periductal fibrosis, biliary intraepithelial neoplasia, and even cholangiocarcinoma. Despite numerous studies at the pathomorphological level, the systemic response and cellular pathogenesis of these disorders are not well studied. To conduct in-depth research and to gain insights into the mechanism by which O. felineus infection causes precancerous liver lesions, we (i) applied a next-generation-sequencing-based technology (high-throughput mRNA sequencing) to identify differentially expressed genes in the liver of golden hamsters infected with O. felineus at 1 and 3 months postinfection and (ii) verified the most pronounced changes in gene expression by western blotting and immunohistochemistry. A total of 2151 genes were found to be differentially expressed between uninfected and infected hamsters ("infection" factor), whereas 371 genes were differentially expressed when we analyzed "time × infection" interaction. Cluster analysis revealed that sets of activated genes of cellular pathways were different between acute (1 month postinfection) and chronic (3 months postinfection) opisthorchiasis. This enriched KEGG pathways were "Cell adhesion molecules", "Hippo signaling", "ECM-receptor interaction", "Cell cycle", "TGF-beta", and "P53 signaling". Moreover, epithelial-mesenchymal transition was the most enriched (q-value = 2.2E-07) MSigDB hallmark in the set of differentially expressed genes of all O. felineus-infected animals. Transcriptomic data were supported by the results of western blotting and immunohistochemistry revealing the upregulation of vimentin, N-cadherin, and α-smooth muscle actin postinfection. Our data expand knowledge about global changes in gene expression in the O. felineus-infected host liver and contribute to understanding the biliary neoplasia associated with the liver fluke infection.
Keywords: DEGs, differentially expressed genes; Differentially expressed gene; EMT, epithelial–mesenchymal transition; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes, O. felineus; Liver; Mesocricetus auratus; OF, Opisthorchis felineus; Opisthorchis felineus; hamster.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Opisthorchis felineus infection provokes time-dependent accumulation of oxidative hepatobiliary lesions in the injured hamster liver.PLoS One. 2019 May 14;14(5):e0216757. doi: 10.1371/journal.pone.0216757. eCollection 2019. PLoS One. 2019. PMID: 31086416 Free PMC article.
-
Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model.Carcinogenesis. 2017 Sep 1;38(9):929-937. doi: 10.1093/carcin/bgx042. Carcinogenesis. 2017. PMID: 28910999 Free PMC article.
-
Comparative liver transcriptome analysis in hamsters infected with food-borne trematodes Opisthorchis felineus, Opisthorchis viverrini, or Clonorchis sinensis.PLoS Negl Trop Dis. 2024 Dec 9;18(12):e0012685. doi: 10.1371/journal.pntd.0012685. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39652576 Free PMC article.
-
Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: A review of medical statistics.Parasitol Int. 2017 Aug;66(4):365-371. doi: 10.1016/j.parint.2016.07.010. Epub 2016 Jul 26. Parasitol Int. 2017. PMID: 27474689 Review.
-
Opisthorchiasis and the Microbiome.Adv Parasitol. 2018;102:1-23. doi: 10.1016/bs.apar.2018.07.001. Epub 2018 Aug 17. Adv Parasitol. 2018. PMID: 30442306 Review.
Cited by
-
Liver Fluke-Derived Molecules Accelerate Skin Repair Processes in a Mouse Model of Type 2 Diabetes Mellitus.Int J Mol Sci. 2024 Nov 8;25(22):12002. doi: 10.3390/ijms252212002. Int J Mol Sci. 2024. PMID: 39596069 Free PMC article.
-
Jagged-1/Notch Pathway and Key Transient Markers Involved in Biliary Fibrosis during Opisthorchis felineus Infection.Trop Med Infect Dis. 2022 Nov 9;7(11):364. doi: 10.3390/tropicalmed7110364. Trop Med Infect Dis. 2022. PMID: 36355906 Free PMC article.
-
Wound healing approach based on excretory-secretory product and lysate of liver flukes.Sci Rep. 2022 Dec 14;12(1):21639. doi: 10.1038/s41598-022-26275-y. Sci Rep. 2022. PMID: 36517588 Free PMC article.
-
Fish and Food-Fatale: Food-borne Trematode Opisthorchis viverrini and Cholangiocarcinoma.Helminthologia. 2023 Dec 31;60(4):287-299. doi: 10.2478/helm-2023-0036. eCollection 2023 Dec. Helminthologia. 2023. PMID: 38222491 Free PMC article. Review.
-
Curcumin and Its Supramolecular Complex with Disodium Glycyrrhizinate as Potential Drugs for the Liver Fluke Infection Caused by Opisthorchis felineus.Pathogens. 2023 Jun 9;12(6):819. doi: 10.3390/pathogens12060819. Pathogens. 2023. PMID: 37375509 Free PMC article.
References
-
- Beer S.A. KMK Scientific Press Ltd.; Moscow: 2005. Biology of the Agent of Opisthorchiasis. (in Russian) (in Russian)
-
- Brazhnikova N.A., Tolkaeva M.V. Cancer of liver, biliary tracts and pancreas at chronic opisthorchiasis (in Russian) Bull. Sib. Med. 2002;2:71–77.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

