A sensitive probe for amyloid fibril detection with strong fluorescence and early response
- PMID: 35542196
- PMCID: PMC9080103
- DOI: 10.1039/c8ra00751a
A sensitive probe for amyloid fibril detection with strong fluorescence and early response
Abstract
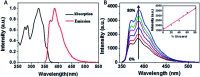
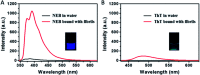
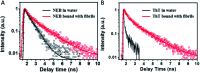
We synthesized a new probe, 4-[2-(2-naphthyl)-(E)-ethenyl]-benzyl(triphenyl)phosphonium bromide (NEB), to detect the formation of amyloid fibrils of bovine insulin. The fluorescence intensity of NEB in the presence of insulin fibrils was 30 times higher than that before fibrillation, with the fluorescence quantum yield increased from 2.5% to 78%. In comparison with the commercially available probe, thioflavin T (ThT), NEB exhibits a 10 times stronger fluorescence and a shorter identification lag phase for detecting insulin fibrillation, indicating a higher sensitivity in detection of insulin oligomers and fibrils.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Interaction of thioflavin T with amyloid fibrils: fluorescence quantum yield of bound dye.J Phys Chem B. 2012 Mar 1;116(8):2538-44. doi: 10.1021/jp2083055. Epub 2012 Feb 15. J Phys Chem B. 2012. PMID: 22268449
-
Steady-state and time-resolved Thioflavin-T fluorescence can report on morphological differences in amyloid fibrils formed by Aβ(1-40) and Aβ(1-42).Biochem Biophys Res Commun. 2015 Mar 6;458(2):418-23. doi: 10.1016/j.bbrc.2015.01.132. Epub 2015 Feb 7. Biochem Biophys Res Commun. 2015. PMID: 25660454
-
[Investigation of the kinetics of insulin amyloid fibrils formation].Tsitologiia. 2013;55(11):809-14. Tsitologiia. 2013. PMID: 25509136 Russian.
-
Fluorescence quantum yield of thioflavin T in rigid isotropic solution and incorporated into the amyloid fibrils.PLoS One. 2010 Oct 29;5(10):e15385. doi: 10.1371/journal.pone.0015385. PLoS One. 2010. PMID: 21048945 Free PMC article.
-
Insulin Formulation Characterization-the Thioflavin T Assays.AAPS J. 2017 Mar;19(2):397-408. doi: 10.1208/s12248-016-0028-6. Epub 2016 Dec 20. AAPS J. 2017. PMID: 28000098 Review.
Cited by
-
Recent Developments of Hybrid Fluorescence Techniques: Advances in Amyloid Detection Methods.Curr Protein Pept Sci. 2024;25(9):667-681. doi: 10.2174/0113892037291597240429094515. Curr Protein Pept Sci. 2024. PMID: 38715332 Review.
References
-
- Chiti F. Dobson C. M. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. - DOI - PubMed
- Dobson C. M. Nature. 2003;426:884–890. doi: 10.1038/nature02261. - DOI - PubMed
- Selkoe D. J. Nature. 2003;426:900–904. doi: 10.1038/nature02264. - DOI - PubMed
- Murphy R. M. Annu. Rev. Biomed. Eng. 2002;4:155–174. doi: 10.1146/annurev.bioeng.4.092801.094202. - DOI - PubMed
-
- Krebs M. R. Bromley E. H. Donald A. M. J. Struct. Biol. 2005;149:30–37. doi: 10.1016/j.jsb.2004.08.002. - DOI - PubMed
- Khurana R. Ionescu-Zanetti C. Pope M. Li J. Nielson L. Ramírez-Alvarado M. Regan L. Fink A. L. Carter S. A. Biophys. J. 2003;85:1135–1144. doi: 10.1016/S0006-3495(03)74550-0. - DOI - PMC - PubMed
- Hwang W. Zhang S. Kamm R. D. Karplus M. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12916–12921. doi: 10.1073/pnas.0402634101. - DOI - PMC - PubMed
-
- Sunde M. Serpell L. C. Bartlam M. Fraser P. E. Pepys M. B. Blake C. C. F. J. Mol. Biol. 1997;273:729–739. doi: 10.1006/jmbi.1997.1348. - DOI - PubMed
- Jiménez J. L. Nettleton E. J. Bouchard M. Robinson C. V. Dobson C. M. Saibil H. R. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9196–9201. doi: 10.1073/pnas.142459399. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

