Convenient chirality transfer from organics to titania: construction and optical properties
- PMID: 35542199
- PMCID: PMC9080238
- DOI: 10.1039/c8ra02926a
Convenient chirality transfer from organics to titania: construction and optical properties
Abstract
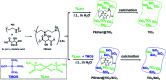
Polyethyleneimine (PEI) complexed with chiral d- (or l-) tartaric acid (tart) in water can self-organize into chiral and crystalline PEI/tart assemblies. It has been previously confirmed that the complexes of PEI/tart could work as catalytic/chiral templates to induce the deposition of SiO2 nanofibres with optical activity but without outwards shape chirality such as helices. In this work, we found that the templating functions of PEI/tart were still effective to prompt the deposition of TiO2 to form chiral PEI/tart@TiO2 hybrid nanofibres under aqueous and room temperature conditions within two hours. Furthermore, the co-deposition of TiO2 and SiO2 was also fulfilled to yield chiral PEI/tart@TiO2/SiO2 nanofibres. These TiO2-containing hybrid nanofibres showed non-helical shapes on the length scale; however, chiroptical signals with mirror relation around the UV-Vis absorption band of TiO2 remarkably appeared on their circular dichroism (CD) spectra. By means of the protocols of XRD, TEM, SEM, UV-Vis, CD and XPS, structural features and thermoproperties of the chiral TiO2 and SiO2/TiO2 were investigated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflicts to declare.
Figures






Similar articles
-
Fascinating chiral information transfer to titania/silica from near to racemic compound self-organized from polyethyleneimine and tartaric acid.Dalton Trans. 2023 Jul 25;52(29):9913-9928. doi: 10.1039/d3dt00652b. Dalton Trans. 2023. PMID: 37401862
-
Chiral SiO2 and Ag@SiO2 Materials Templated by Complexes Consisting of Comblike Polyethyleneimine and Tartaric Acid.Chemistry. 2015 Oct 26;21(44):15667-75. doi: 10.1002/chem.201502290. Epub 2015 Sep 9. Chemistry. 2015. PMID: 26350940
-
Polycondensation and carbonization of phenolic resin on structured nano/chiral silicas: reactions, morphologies and properties.J Mater Chem B. 2016 Jan 28;4(4):626-634. doi: 10.1039/c5tb01966d. Epub 2015 Nov 24. J Mater Chem B. 2016. PMID: 32262944
-
Emergent chiroptical properties in supramolecular and plasmonic assemblies.Chem Soc Rev. 2021 Oct 18;50(20):11208-11226. doi: 10.1039/d0cs01583k. Chem Soc Rev. 2021. PMID: 34522920 Review.
-
Chiral Perovskites for Next-Generation Photonics: From Chirality Transfer to Chiroptical Activity.Adv Mater. 2021 Nov;33(47):e2005760. doi: 10.1002/adma.202005760. Epub 2021 Apr 22. Adv Mater. 2021. PMID: 33885185 Review.
References
-
- Govan J. and Gun'ko Y. K., in Nanoscience, The Royal Society of Chemistry, vol. 3, 2016, pp. 1–30
-
- Chen C. Shi H. Zhao G. J. Phys. Chem. C. 2014;118:12041–12049. - PubMed
LinkOut - more resources
Full Text Sources

