Therapeutic polymeric nanomedicine: GSH-responsive release promotes drug release for cancer synergistic chemotherapy
- PMID: 35542287
- PMCID: PMC9075505
- DOI: 10.1039/c9ra07051f
Therapeutic polymeric nanomedicine: GSH-responsive release promotes drug release for cancer synergistic chemotherapy
Abstract
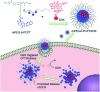
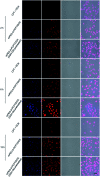
To obtain an efficient dual-drug release and enhance therapeutic efficiency for combination chemotherapy, a glutathione (GSH)-responsive therapeutic amphiphilic polyprodrug copolymer (mPEG-b-PCPT) is synthesized to load doxorubicin (DOX) via hydrophobic and π-π stacking interaction. In this nanomedicine system (mPEG-b-PCPT/DOX), the ratio of the two drugs can be easily modulated by changing the loading content of DOX. The in vitro drug release curves and laser confocal images suggested that the release of CPT and DOX is induced through a "release promotes release strategy": after internalization into tumor cells, the disulfide bonds in the nanomedicine are cleaved by glutathione (GSH) in the cytoplasm and then lead to the release of CPT. Meanwhile, the disassembly of nanomedicine immediately promotes the co-release of DOX. The optimum dose ratio of CPT and DOX is evaluated via the combination index (CI) value using HepG-2 cells. The results of cell apoptosis and cell viability prove the better synergistic efficiency of the nanomedicine than free drugs at the optimum dose ratio of 1. Consequently, this stimuli-responsive synergistic chemotherapy system provides a direction for the fabrication of nanomedicines possessing promising potential in clinical trials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
GSH/pH dual-responsive biodegradable camptothecin polymeric prodrugs combined with doxorubicin for synergistic anticancer efficiency.Biomater Sci. 2019 Aug 1;7(8):3277-3286. doi: 10.1039/c9bm00425d. Epub 2019 Jun 10. Biomater Sci. 2019. PMID: 31180396
-
Glutathione-triggered dual release of doxorubicin and camptothecin for highly efficient synergistic anticancer therapy.Colloids Surf B Biointerfaces. 2018 Sep 1;169:273-279. doi: 10.1016/j.colsurfb.2018.05.025. Epub 2018 May 17. Colloids Surf B Biointerfaces. 2018. PMID: 29787951
-
TME-Responsive Polyprodrug Micelles for Multistage Delivery of Doxorubicin with Improved Cancer Therapeutic Efficacy in Rodents.Adv Healthc Mater. 2020 Sep;9(18):e2000387. doi: 10.1002/adhm.202000387. Epub 2020 Aug 20. Adv Healthc Mater. 2020. PMID: 32815646
-
A glutathione-responsive sulfur dioxide polymer prodrug as a nanocarrier for combating drug-resistance in cancer chemotherapy.Biomaterials. 2018 Sep;178:706-719. doi: 10.1016/j.biomaterials.2018.02.011. Epub 2018 Feb 3. Biomaterials. 2018. PMID: 29433753
-
Fabrication of pH/Redox Dual-Responsive Mixed Polyprodrug Micelles for Improving Cancer Chemotherapy.Front Pharmacol. 2022 Feb 3;12:802785. doi: 10.3389/fphar.2021.802785. eCollection 2021. Front Pharmacol. 2022. PMID: 35185545 Free PMC article.
Cited by
-
Nanotechnology-based combinatorial phototherapy for enhanced cancer treatment.RSC Adv. 2022 Apr 4;12(16):9725-9737. doi: 10.1039/d1ra09067d. eCollection 2022 Mar 25. RSC Adv. 2022. PMID: 35424935 Free PMC article. Review.
-
An NIF-doped ZIF-8 hybrid membrane for continuous antimicrobial treatment.RSC Adv. 2020 Feb 19;10(13):7360-7367. doi: 10.1039/d0ra00108b. eCollection 2020 Feb 18. RSC Adv. 2020. PMID: 35492192 Free PMC article.
-
Towards precision medicine using biochemically triggered cleavable conjugation.Commun Chem. 2025 Apr 2;8(1):100. doi: 10.1038/s42004-025-01491-5. Commun Chem. 2025. PMID: 40175511 Free PMC article. Review.
-
Intranasal Administration of Catechol-Based Pt(IV) Coordination Polymer Nanoparticles for Glioblastoma Therapy.Nanomaterials (Basel). 2022 Apr 5;12(7):1221. doi: 10.3390/nano12071221. Nanomaterials (Basel). 2022. PMID: 35407338 Free PMC article.
-
Drug self-delivery systems: A comprehensive review on small molecule nanodrugs.Bioimpacts. 2024 Oct 27;15:30161. doi: 10.34172/bi.30161. eCollection 2025. Bioimpacts. 2024. PMID: 40161942 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

