Fabrication and photocatalytic property of magnetic SrTiO3/NiFe2O4 heterojunction nanocomposites
- PMID: 35542406
- PMCID: PMC9078118
- DOI: 10.1039/c7ra12393k
Fabrication and photocatalytic property of magnetic SrTiO3/NiFe2O4 heterojunction nanocomposites
Abstract
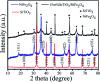
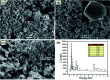
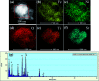
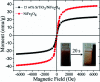
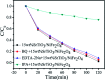
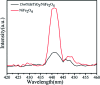
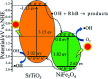
Novel multifunctional SrTiO3/NiFe2O4 nanocomposites were successfully fabricated via a two-step route. The as-prepared samples were characterized by using X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), field-emission transmission electron microscopy (TEM), UV-visible diffuse reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) spectroscopy and vibrating sample magnetometry (VSM). The results indicate that the SrTiO3/NiFe2O4 heterostructures are composed of SrTiO3 spheroidal nanoparticles adhered to NiFe2O4 polyhedra. The heterojunction established in the composite material accelerates the process of electron-hole pair separation and boosts the photo-Fenton reaction. Among the samples, 15 wt% SrTiO3/NiFe2O4 nanocomposites exhibit a powerful light response and excellent room temperature ferromagnetism. Subsequently, the photocatalytic degradation of RhB over the as-prepared samples was investigated and optimized, revealing that the 15 wt% SrTiO3/NiFe2O4 nanocomposites exhibit the best photocatalytic activity and stability under simulated solar light irradiation. Furthermore, according to experimental results, the possible mechanism of improved photocatalytic activity was also proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Ahuja A. Mosalam K. M. Energy and Buildings. 2017;153:448–460. doi: 10.1016/j.enbuild.2017.06.062. - DOI
-
- Piskunov S. Lisovski O. Begens J. Bocharov D. Zhukovskii Y. F. Wessel M. Spohr E. J. Phys. Chem. C. 2015;119:18686–18696.
-
- Cherian C. T. Sundaramurthy J. Reddy M. V. Kumar P. S. Mani K. Pliszka D. Sow C. H. Ramakrishna S. Chowdari B. V. R. ACS Appl. Mater. Interfaces. 2013;5:9957–9963. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

