Biological properties of calcium phosphate biomaterials for bone repair: a review
- PMID: 35542623
- PMCID: PMC9077253
- DOI: 10.1039/c7ra11278e
Biological properties of calcium phosphate biomaterials for bone repair: a review
Abstract
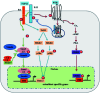
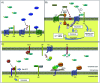
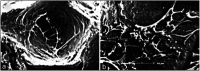
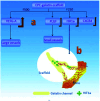
Bone defects are a common disease threatening the health of many people. Calcium phosphate (CaP) is an ideal bone substitutive material that is widely used for bone repair due to its excellent biological properties including osteoinductivity, osteoconductivity and biodegradability. For this reason, investigation of these properties and the effects of various influencing factors is vital for modulating calcium phosphate during the design process to maximally satisfy clinical requirements. In this study, the latest studies on the biological properties of CaP biomaterials, including hydroxyapatite (HA), tricalcium phosphate (TCP), and biphasic calcium phosphate (BCP), have been summarized. Moreover, recent advances on how these properties are altered by different factors are reviewed. Considering the limited mechanical strength of CaP materials, this study also reviews CaP composites with different materials as improvement measures. Finally, perspectives regarding future developments of CaP materials are also provided.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
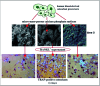

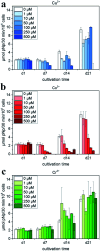

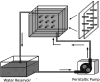

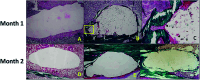



References
-
- Shao R. Quan R. Zhang L. Wei X. Yang D. Xie S. J. Ceram. Soc. Jpn. 2015;123:17–20. doi: 10.2109/jcersj2.123.17. - DOI
-
- Liu C. Tang R. Chin. J. Inorg. Chem. 2014;30:1–9.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

