Evaluation of anti α-d-Glc p-(1→4)-α-d-Glc p (GAGA4) IgM antibodies as a biomarker for multiple sclerosis
- PMID: 35542693
- PMCID: PMC9084297
- DOI: 10.1039/c8ra04897e
Evaluation of anti α-d-Glc p-(1→4)-α-d-Glc p (GAGA4) IgM antibodies as a biomarker for multiple sclerosis
Abstract
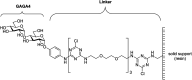
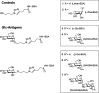
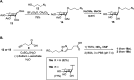
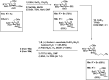
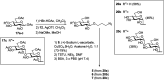
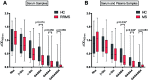
The correct diagnosis of multiple sclerosis (MS) remains challenging due to the complex pathophysiological and clinical characteristics of the disease. Consequently, there has been immense interest in finding a non-invasive diagnostic test for MS. Recent studies found that serum anti-α-d-Glcp-(1→4)-α-d-Glcp (GAGA4) IgM antibodies were upregulated in MS patients, and this finding led to the development of a commercial diagnostic test (gMS® Dx test), although the test has poor selectivity and has not been independently validated. Herein, we developed an enzyme-linked immunosorbent assay (ELISA) to evaluate the use and reliability of several anti-glucose IgM antibodies, including those against GAGA4, as diagnostic biomarkers for MS. In contrast to previous studies, our results show that serum anti-GAGA4 IgM antibody levels are not significantly higher in MS patients, which could potentially explain the poor selectivity of the commercial test.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Serum anti-GAGA4 IgM antibodies differentiate relapsing remitting and secondary progressive multiple sclerosis from primary progressive multiple sclerosis and other neurological diseases.J Neuroimmunol. 2009 Dec 10;217(1-2):95-101. doi: 10.1016/j.jneuroim.2009.07.017. Epub 2009 Oct 30. J Neuroimmunol. 2009. PMID: 19879655
-
Anti-alpha-glucose-based glycan IgM antibodies predict relapse activity in multiple sclerosis after the first neurological event.Mult Scler. 2009 Apr;15(4):422-30. doi: 10.1177/1352458508101944. Mult Scler. 2009. PMID: 19324980 Free PMC article.
-
Predictive nature of IgM anti-α-glucose serum biomarker for relapse activity and EDSS progression in CIS patients: a BENEFIT study analysis.Mult Scler. 2012 Jul;18(7):966-73. doi: 10.1177/1352458511432327. Epub 2011 Dec 19. Mult Scler. 2012. PMID: 22183938 Free PMC article. Clinical Trial.
-
Serum anti-Glc(alpha1,4)Glc(alpha) antibodies as a biomarker for relapsing-remitting multiple sclerosis.J Neurol Sci. 2006 May 15;244(1-2):59-68. doi: 10.1016/j.jns.2005.12.006. Epub 2006 Feb 9. J Neurol Sci. 2006. PMID: 16480743
-
Low reliability of anti-KIR4.183-120 peptide auto-antibodies in multiple sclerosis patients.Mult Scler. 2018 Jun;24(7):910-918. doi: 10.1177/1352458517711275. Epub 2017 May 26. Mult Scler. 2018. PMID: 28548026
References
LinkOut - more resources
Full Text Sources

