Sustainable inverse-vulcanised sulfur polymers
- PMID: 35542731
- PMCID: PMC9083557
- DOI: 10.1039/c8ra04446e
Sustainable inverse-vulcanised sulfur polymers
Erratum in
-
Correction: Sustainable inverse-vulcanised sulfur polymers.RSC Adv. 2018 Aug 29;8(53):30429. doi: 10.1039/c8ra90071j. eCollection 2018 Aug 24. RSC Adv. 2018. PMID: 35546828 Free PMC article.
Abstract
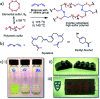
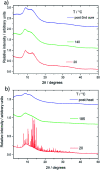
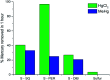
We demonstrate two renewable crosslinkers that can stabilise sustainable high sulfur content polymers, via inverse-vulcanisation. With increasing levels of sulfur produced as a waste byproduct from hydrodesulfurisation of crude oil and gas, the need to find a method to utilise this abundant feedstock is pressing. The resulting sulfur copolymers can be synthesised relatively quickly, using a one-pot solvent free method, producing polymeric materials that are shape-persistent solids at room temperature and compare well to other inverse vulcanised polymers. The physical properties of these high sulfur polymeric materials, coupled with the ability to produce them sustainably, allow broad potential utility.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Kutney G., Sulfur: history, technology, applications & industry, ChemTec Publishing, Toronto, 2nd edn, 2013, vol. 2013
-
- Blight L., Currell B. R., Nash B. J., Scott R. A. M. and Stillo C., in New Uses of Sulfur—II, American Chemical Society, 1978, ch. 2, vol. 165, pp. 13–30
-
- Steudel R., Elemental Sulfur and Sulfur-Rich Compounds I, Springer Berlin/Heidelberg, 2003, vol. 2003
-
- Worthington M. J. H. Kucera R. L. Chalker J. M. Green Chem. 2017;19:2748–2761. doi: 10.1039/C7GC00014F. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

