Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications
- PMID: 35542741
- PMCID: PMC9083935
- DOI: 10.1039/c8ra03861a
Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications
Abstract
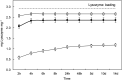
The aim of this work was to immobilize antimicrobial peptides onto a fibrous scaffold to create functional wound dressings. The scaffold was produced by electrospinning from a mixture of the water soluble polymers poly(acrylic acid) and poly(vinyl alcohol) and subsequently heat cured at 140 °C to produce a stable material with fibre diameter below micron size. The peptides were incorporated into the negatively charged scaffold by electrostatic interaction. The best results were obtained for lysozyme impregnated at pH 7, which rendered a loading of up to 3.0 × 10-4 mmol mg-1. The dressings were characterized using SEM, ATR-FTIR, elemental analysis, ζ-potential and confocal microscopy using fluorescamine as an amine-reactive probe. The dressings preserved their fibrous structure after impregnation and peptides were distributed homogeneously throughout the fibrous network. The antibacterial activity was assessed by solid agar diffusion tests and growth inhibition in liquid cultures using Staphylococcus aureus, a pathogenic strain generally found in infected wounds. The antibacterial activity caused clear halo inhibition zones for lysozyme-loaded dressings and a 4-fold decrease in S. aureus viable colonies after two weeks of contact of dressings with bacterial liquid cultures. The release profile in different media showed sustained release in acidic environments, and a rapid discharge at high pH values. The incorporation of lysozyme resulted in dressing surfaces essentially free of microbial growth after 14 days of contact with bacteria at pH 7.4 attributed to the peptide that remained attached to the dressing surface.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

