Fe3O4@SiO2 nanoparticle supported ionic liquid for green synthesis of antibacterially active 1-carbamoyl-1-phenylureas in water
- PMID: 35542743
- PMCID: PMC9083804
- DOI: 10.1039/c8ra04368j
Fe3O4@SiO2 nanoparticle supported ionic liquid for green synthesis of antibacterially active 1-carbamoyl-1-phenylureas in water
Abstract
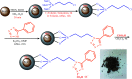
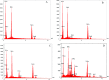
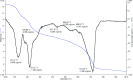
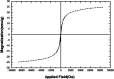
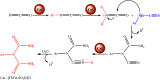
In the present work, we have designed a novel, heterogeneous and recyclable magnetic Brønsted acidic ionic liquid based on 5-phenyl-1H-tetrazole. The {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/Cl} ([FSTet-SO3H]Cl) was prepared via the immobilization of 5-phenyl-1H-tetrazole-bonded sulfonic acid onto the surface of silica-coated magnetic nanoparticles using 3-chloropropyltriethoxysilane as a linker. The catalyst was characterized by XRD, TEM, FESEM, EDS, TG-DTA, and FT-IR. The ability and high activity of this catalyst were demonstrated in the synthesis of 1-carbamoyl-1-phenylureas with good to excellent yields via a new, simple and one-pot procedure in aqueous media under reflux conditions. This procedure has advantages such as high yields, short reaction times, a simple methodology and work-up process, green reaction conditions, high stability, catalytic activity, and easy preparation, separation and reusability of the catalyst. The synthesis of these compounds was confirmed by FT-IR, 1H NMR, 13C NMR and CHN. In addition, we investigated the biological properties of the 1-carbamoyl-1-phenylureas as newly synthesized compounds. The described catalyst could be easily separated from the reaction mixture by additional magnetic force and reused several times without a remarkable loss of its catalytic activity and any considerable changes in the product yield and the reaction time.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures














References
-
- Marsh K. N. Boxall J. A. Lichtenthaler R. Fluid Phase Equilib. 2004;219:93. doi: 10.1016/j.fluid.2004.02.003. - DOI
-
- Bentivoglio G. Röder T. Fasching M. Buchberger M. Schottenberger H. Sixta H. Lenzinger Ber. 2006;86:154.
-
- Welton T. Chem. Rev. 1999;99(8):2071. doi: 10.1021/cr980032t. - DOI - PubMed
- Yue C. Fang D. Liu L. Yi T. F. J. Mol. Liq. 2011;163:99. doi: 10.1016/j.molliq.2011.09.001. - DOI
- Greaves T. L. Drummond C. J. Chem. Rev. 2008;108:206. doi: 10.1021/cr068040u. - DOI - PubMed
- Hallett J. P. Welton T. Chem. Rev. 2011;111:3508. doi: 10.1021/cr1003248. - DOI - PubMed
-
- Jonathan G. H. Heather D. W. Richard P. S. Ann E. V. Robin D. R. Chem. Commun. 1998;16:1765.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

