Role of ceria in the improvement of NO removal of lanthanum-based perovskite-type catalysts
- PMID: 35542810
- PMCID: PMC9079312
- DOI: 10.1039/c8ra00456k
Role of ceria in the improvement of NO removal of lanthanum-based perovskite-type catalysts
Abstract
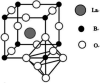
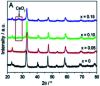
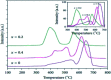
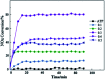
Lanthanum-based perovskite-type oxides represented by LaBO3 (B = Co, Fe, Mn) have been thought to present strong limitations for practical application although they are active for catalytic removal of NO. Cerium (Ce) substitution has been extensively studied to modify the properties of perovskites. It is noted that a new phase of ceria (CeO2) can be separated from perovskites when the doping ratio exceeds the solution limit (x > S). This review outlines the relationship between the existence of CeO2 phase and catalytic activity. CeO2 dispersing on the lattice surface or small particles are beneficial for catalytic activity, but larger particles are adverse. Ce-doped LaBO3 perovskites exhibiting the best activity must contain additional CeO2 phases. In addition, CeO2-supported LaBO3 perovskite catalysts are discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures
Similar articles
-
Design Aspects of Doped CeO2 for Low-Temperature Catalytic CO Oxidation: Transient Kinetics and DFT Approach.ACS Appl Mater Interfaces. 2021 May 19;13(19):22391-22415. doi: 10.1021/acsami.1c02934. Epub 2021 Apr 9. ACS Appl Mater Interfaces. 2021. PMID: 33834768 Free PMC article.
-
CeO2 supported MOFs derived LaBxFeyO3 (B=Mn, Co) perovskite catalysts for degradation of toluene.Environ Sci Pollut Res Int. 2023 Mar;30(15):45414-45427. doi: 10.1007/s11356-023-25610-8. Epub 2023 Jan 27. Environ Sci Pollut Res Int. 2023. PMID: 36707473
-
Catalytic oxidation of ethyl acetate over LaBO3 (B = Co, Mn, Ni, Fe) perovskites supported silver catalysts.RSC Adv. 2018 Sep 27;8(58):33425-33431. doi: 10.1039/c8ra06933f. eCollection 2018 Sep 24. RSC Adv. 2018. PMID: 35548126 Free PMC article.
-
An Insight into Geometries and Catalytic Applications of CeO2 from a DFT Outlook.Molecules. 2021 Oct 27;26(21):6485. doi: 10.3390/molecules26216485. Molecules. 2021. PMID: 34770889 Free PMC article. Review.
-
Recent advances and perspectives of CeO2-based catalysts: Electronic properties and applications for energy storage and conversion.Front Chem. 2022 Dec 8;10:1089708. doi: 10.3389/fchem.2022.1089708. eCollection 2022. Front Chem. 2022. PMID: 36569964 Free PMC article. Review.
Cited by
-
Structure, thermostability and magnetic properties of cubic Ce2-x Ti2O7 pyrochlore obtained via sol-gel preparation.RSC Adv. 2022 May 30;12(24):15348-15353. doi: 10.1039/d2ra01714h. eCollection 2022 May 17. RSC Adv. 2022. PMID: 35734022 Free PMC article.
-
DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface.Materials (Basel). 2019 Apr 28;12(9):1379. doi: 10.3390/ma12091379. Materials (Basel). 2019. PMID: 31035353 Free PMC article.
-
Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions.Materials (Basel). 2023 Jan 29;16(3):1142. doi: 10.3390/ma16031142. Materials (Basel). 2023. PMID: 36770148 Free PMC article.
References
-
- Li J. Wang S. Zhou L. Luo G. Wei F. Chem. Eng. J. 2014;255:126–133. doi: 10.1016/j.cej.2014.06.015. - DOI
-
- Li M. Easterling V. G. Harold M. P. Appl. Catal., B. 2016;184:364–380. doi: 10.1016/j.apcatb.2015.11.029. - DOI
-
- Imanaka N. Masui T. Appl. Catal., A. 2012;431:1–8. doi: 10.1016/j.apcata.2012.02.047. - DOI
-
- Fritz A. Pitchon V. Appl. Catal., B. 1997;13:1–25. doi: 10.1016/S0926-3373(96)00102-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

