Insights into a novel class of azobenzenes incorporating 4,6- O-protected sugars as photo-responsive organogelators
- PMID: 35542832
- PMCID: PMC9076560
- DOI: 10.1039/c9ra08033c
Insights into a novel class of azobenzenes incorporating 4,6- O-protected sugars as photo-responsive organogelators
Abstract
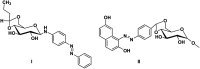
A novel class of 4,6-O-butylidene/ethylidene/benzylidene β-d-glucopyranose gelator functionalized with photo-responsive azobenzene moieties were designed and synthesized and also characterized using different spectral techniques. These azobenzene-based organogelators can gel even at lower concentrations (critical gelation concentration - 0.5% and 1%). A morphological study of the gels shows one-dimensional aggregated bundles and helical fibres. The main driving force for the self-assembly is through cooperative interactions exhibited by the different groups viz., sugar hydroxyl (hydrogen bonding interaction), azobenzene (aromatic π-π interaction) and alkyl chain of the protecting group (van der Waals interaction).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Ajayaghosh A. Vijayakumar C. Varghese R. George S. Angew. Chem., Int. Ed. 2006;45:456. doi: 10.1002/anie.200503258. - DOI - PubMed
- Sugiyasu K. Fujita N. Shinkai S. Angew. Chem., Int. Ed. 2004;43:1229. doi: 10.1002/anie.200352458. - DOI - PubMed
- George M. Weiss R. G. Acc. Chem. Res. 2006;39:489. doi: 10.1021/ar0500923. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

