Synthesis of C14-C21 acid fragments of cytochalasin Z8 via anti-selective aldol condensation and B-alkyl Suzuki-Miyaura cross-coupling
- PMID: 35542899
- PMCID: PMC9077782
- DOI: 10.1039/c7ra13391j
Synthesis of C14-C21 acid fragments of cytochalasin Z8 via anti-selective aldol condensation and B-alkyl Suzuki-Miyaura cross-coupling
Erratum in
-
Correction: Synthesis of C14-C21 acid fragments of cytochalasin Z8 via anti-selective aldol condensation and B-alkyl Suzuki-Miyaura cross-coupling.RSC Adv. 2018 Feb 21;8(15):8246. doi: 10.1039/c8ra90018c. eCollection 2018 Feb 19. RSC Adv. 2018. PMID: 35543933 Free PMC article.
Abstract
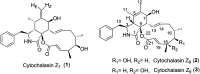
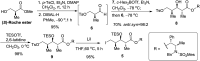
An efficient synthesis of the C14-C21 acid fragment of cytochalasin Z8 was accomplished in 10 steps with 14% overall yield. Boron-mediated anti-selective aldol condensation and Pd(OAc)2-Aphos-Y-catalysed B-alkyl Suzuki-Miyaura cross-coupling were employed to construct the requisite C17 and C18 stereogenic centres and alkene subunit.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Binder M. Tamm C. Angew. Chem., Int. Ed. 1973;12:370. doi: 10.1002/anie.197303701. - DOI - PubMed
- Wang F. Wei H. Zhu T. Li D. Lin Z. Gu Q. Chem. Biodiversity. 2011;8:887. doi: 10.1002/cbdv.201000133. - DOI - PubMed
- Wang J. Wang Z. Ju Z. Wan J. Liao S. Lin X. Zhang T. Zhou X. Chen H. Tu Z. Planta Med. 2015;81:160. doi: 10.1055/s-0034-1383403. - DOI - PubMed
- Xu J. RSC Adv. 2015;5:841. doi: 10.1039/C4RA11756E. - DOI
-
- Stork G. Nakamura E. J. Am. Chem. Soc. 1983;105:5510. doi: 10.1021/ja00354a072. - DOI
- Dyke H. Sauter R. Steel P. Thomas E. J. J. Chem. Soc., Chem. Commun. 1986:1447. doi: 10.1039/C39860001447. - DOI
- Thomas E. J. Whitehead J. W. F. J. Chem. Soc., Chem. Commun. 1986:727. doi: 10.1039/C39860000727. - DOI
- Thomas E. J. Whitehead J. W. F. J. Chem. Soc., Perkin Trans. 1. 1989:499. doi: 10.1039/P19890000499. - DOI
- Merifield E. Thomas E. J. J. Chem. Soc., Chem. Commun. 1990:464. doi: 10.1039/C39900000464. - DOI
- Merifield E. Thomas E. J. J. Chem. Soc., Perkin Trans. 1. 1999:3269. doi: 10.1039/A906412E. - DOI
-
- Stork G. Nakahara Y. Nakahara Y. Greenlee W. J. J. Am. Chem. Soc. 1978;100:7775. doi: 10.1021/ja00492a082. - DOI
- Vedejs E. Reid J. G. J. Am. Chem. Soc. 1984;106:4617. doi: 10.1021/ja00328a053. - DOI
- Vedejs E. Rodgers J. D. Wittenberger S. J. J. Am. Chem. Soc. 1988;110:4822. doi: 10.1021/ja00222a047. - DOI
- Trost B. M. Ohmori M. Boyd S. A. Okawara H. Brickner S. J. J. Am. Chem. Soc. 1989;111:8281. doi: 10.1021/ja00203a042. - DOI
- Vedejs E. Reid J. G. Rodgers J. D. Wittenberger S. J. J. Am. Chem. Soc. 1990;112:4351. doi: 10.1021/ja00167a036. - DOI
LinkOut - more resources
Full Text Sources

