Assessment of inhalation toxicity of cigarette smoke and aerosols from flavor mixtures: 5-week study in A/J mice
- PMID: 35543240
- PMCID: PMC9545811
- DOI: 10.1002/jat.4338
Assessment of inhalation toxicity of cigarette smoke and aerosols from flavor mixtures: 5-week study in A/J mice
Abstract
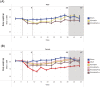
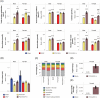
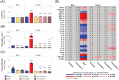
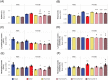
Most flavors used in e-liquids are generally recognized as safe for oral consumption, but their potential effects when inhaled are not well characterized. In vivo inhalation studies of flavor ingredients in e-liquids are scarce. A structure-based grouping approach was used to select 38 flavor group representatives (FGR) on the basis of known and in silico-predicted toxicological data. These FGRs were combined to create prototype e-liquid formulations and tested against cigarette smoke (CS) in a 5-week inhalation study. Female A/J mice were whole-body exposed for 6 h/day, 5 days/week, for 5 weeks to air, mainstream CS, or aerosols from (1) test formulations containing propylene glycol (PG), vegetable glycerol (VG), nicotine (N; 2% w/w), and flavor (F) mixtures at low (4.6% w/w), medium (9.3% w/w), or high (18.6% w/w) concentration or (2) base formulation (PG/VG/N). Male A/J mice were exposed to air, PG/VG/N, or PG/VG/N/F-high under the same exposure regimen. There were no significant mortality or in-life clinical findings in the treatment groups, with only transient weight loss during the early exposure adaptation period. While exposure to flavor aerosols did not cause notable lung inflammation, it caused only minimal adaptive changes in the larynx and nasal epithelia. In contrast, exposure to CS resulted in lung inflammation and moderate-to-severe changes in the epithelia of the nose, larynx, and trachea. In summary, the study evaluates an approach for assessing the inhalation toxicity potential of flavor mixtures, thereby informing the selection of flavor exposure concentrations (up to 18.6%) for a future chronic inhalation study.
Keywords: E-cigarette; emphysema; flavor toolbox; harm reduction; inflammation; inhalation.
© 2022 The Authors. Journal of Applied Toxicology published by John Wiley & Sons Ltd.
Conflict of interest statement
The flavor groups representatives (FGR) used in this study were developed on the basis of individual flavor chemicals used at PMI and ALCS. All authors are (or were) employees of PMI or ALCS or worked with PMI under contractual agreement.
Figures
Similar articles
-
Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints.Food Chem Toxicol. 2017 Nov;109(Pt 1):315-332. doi: 10.1016/j.fct.2017.09.001. Epub 2017 Sep 5. Food Chem Toxicol. 2017. PMID: 28882640
-
Conventional and multi-omics assessments of subacute inhalation toxicity due to propylene glycol and vegetable glycerin aerosol produced by electronic cigarettes.Ecotoxicol Environ Saf. 2024 Feb;271:116002. doi: 10.1016/j.ecoenv.2024.116002. Epub 2024 Jan 26. Ecotoxicol Environ Saf. 2024. PMID: 38277972
-
Toxicologic evaluation of humectants added to cigarette tobacco: 13-week smoke inhalation study of glycerin and propylene glycol in Fischer 344 rats.Inhal Toxicol. 2002 Nov;14(11):1135-52. doi: 10.1080/08958370290084827. Inhal Toxicol. 2002. PMID: 12454795
-
A 6-month inhalation toxicology study in Apoe-/- mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract.Arch Toxicol. 2021 May;95(5):1805-1829. doi: 10.1007/s00204-021-03020-4. Epub 2021 May 7. Arch Toxicol. 2021. PMID: 33963423 Free PMC article.
-
Electronic cigarette aerosol increases the risk of organ dysfunction by enhancing oxidative stress and inflammation.Drug Chem Toxicol. 2022 Nov;45(6):2561-2567. doi: 10.1080/01480545.2021.1972680. Epub 2021 Sep 2. Drug Chem Toxicol. 2022. PMID: 34474637 Review.
Cited by
-
Flavoured Vaping Products in Tobacco Harm Reduction: A Regulatory Perspective.Cureus. 2025 Aug 1;17(8):e89196. doi: 10.7759/cureus.89196. eCollection 2025 Aug. Cureus. 2025. PMID: 40757083 Free PMC article. Review.
-
Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices.Am J Physiol Lung Cell Mol Physiol. 2023 Mar 1;324(3):L345-L357. doi: 10.1152/ajplung.00222.2022. Epub 2023 Jan 24. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36692165 Free PMC article.
-
Chemical and in vitro toxicological comparison of emissions from a heated tobacco product and the 1R6F reference cigarette.Toxicol Rep. 2023 Feb 15;10:281-292. doi: 10.1016/j.toxrep.2023.02.005. eCollection 2023. Toxicol Rep. 2023. PMID: 36876026 Free PMC article.
-
The Toxicological Effects of e-Cigarette Use in the Upper Airway: A Scoping Review.Otolaryngol Head Neck Surg. 2024 May;170(5):1246-1269. doi: 10.1002/ohn.652. Epub 2024 Feb 14. Otolaryngol Head Neck Surg. 2024. PMID: 38353408 Free PMC article.
-
In Vitro assessments of ENDS toxicity in the respiratory tract: Are we there yet?NAM J. 2025;1:100016. doi: 10.1016/j.namjnl.2025.100016. Epub 2025 Apr 3. NAM J. 2025. PMID: 40264558 Free PMC article.
References
-
- Action on Smoking and Health . (2021). Use of e‐cigarettes (vapes) among adults in Great Britain. Retrieved from https://ash.org.uk/wp-content/uploads/2021/06/Use-of-e-cigarettes-vapes-...
-
- Asgharian, B. , Price, O. T. , Oldham, M. , Chen, L. C. , Saunders, E. L. , Gordon, T. , Mikheev, V. B. , Minard, K. R. , & Teeguarden, J. G. (2014). Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhalation Toxicology, 26(14), 829–842. 10.3109/08958378.2014.935535 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

