A Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine With and Without Adjuvant in Healthy Older Adults
- PMID: 35543281
- PMCID: PMC9749002
- DOI: 10.1093/infdis/jiac189
A Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine With and Without Adjuvant in Healthy Older Adults
Abstract
Background: Respiratory syncytial virus (RSV) is an important cause of disease in older adults. We evaluated the safety and immunogenicity of a stabilized RSV prefusion F subunit (RSVpreF) vaccine candidate with/without adjuvant in adults aged 65-85 years.
Methods: Primary cohort participants were equally randomized to 1 of 7 RSVpreF formulations: 60 µg with either Al(OH)3 or CpG/Al(OH)3, 120 µg with either Al(OH)3 or CpG/Al(OH)3, 240 µg with either Al(OH)3 or CpG/Al(OH)3, 240 µg unadjuvanted, or placebo, administered concomitantly with high-dose seasonal inactivated influenza vaccine (SIIV). Participants in the month 0,2 cohort were randomized to RSVpreF 240 µg with CpG/Al(OH)3 or placebo, administered at months 0 and 2.
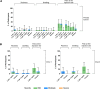
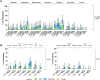
Results: All RSVpreF vaccine candidates elicited robust and persistent serum neutralizing responses when administered alone or with SIIV. There was no notable difference in neutralizing response between the formulations, including those containing CpG. In the month 0,2 cohort, there was no booster effect of dose 2. SIIV responses were similar or slightly lower with concomitant administration of RSVpreF. Most systemic and local reactions were mild and more frequent after RSVpreF than placebo.
Conclusions: RSVpreF formulations were well tolerated and elicited robust neutralizing responses in older adults; however, CpG/Al(OH)3 did not further enhance responses. Clinical Trials Registration. NCT03572062.
Keywords: RSV; prefusion F subunit; respiratory syncytial virus; vaccine.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . J. B., A. J., Q. J., K. S., D. C., M. M., J. L., A. G., K. J., W. G., P. D., and B. S.-T. are employees of Pfizer Inc and may hold Pfizer Inc stock or stock options. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Hall CB, Weinberg GA, Blumkin AK, et al. . Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. - PubMed
-
- Centers for Disease Control and Prevention . Respiratory syncytial virus infection (RSV). Trends and surveillance. https://www.cdc.gov/rsv/research/us-surveillance.html. Accessed 02 November 2020.

