A PLETHORA/PIN-FORMED/auxin network mediates prehaustorium formation in the parasitic plant Striga hermonthica
- PMID: 35543497
- PMCID: PMC9342978
- DOI: 10.1093/plphys/kiac215
A PLETHORA/PIN-FORMED/auxin network mediates prehaustorium formation in the parasitic plant Striga hermonthica
Abstract
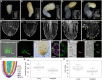
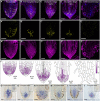
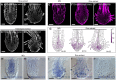
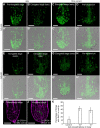
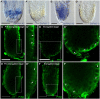
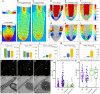
The parasitic plant Striga (Striga hermonthica) invades the host root through the formation of a haustorium and has detrimental impacts on cereal crops. The haustorium results from the prehaustorium, which is derived directly from the differentiation of the Striga radicle. The molecular mechanisms leading to radicle differentiation shortly after germination remain unclear. In this study, we determined the developmental programs that regulate terminal prehaustorium formation in S. hermonthica at cellular resolution. We showed that shortly after germination, cells in the root meristem undergo multiplanar divisions. During growth, the meristematic activity declines and associates with reduced expression of the stem cell regulator PLETHORA1 and the cell cycle genes CYCLINB1 and HISTONE H4. We also observed a basal localization of the PIN-FORMED (PIN) proteins and a decrease in auxin levels in the meristem. Using the structural layout of the root meristem and the polarity of outer-membrane PIN proteins, we constructed a mathematical model of auxin transport that explains the auxin distribution patterns observed during S. hermonthica root growth. Our results reveal a fundamental molecular and cellular framework governing the switch of S. hermonthica roots to form the invasive prehaustoria.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures
References
-
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The P LET HORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 9: 109–120 - PubMed
-
- Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel plant hormone. Annu Rev Plant Biol 66: 161–186 - PubMed
-
- Baird W V., Riopel JL (1983) Experimental studies of the attachment of the parasitic angiosperm Agalinis purpurea to a host. Protoplasma 118: 206–218
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

