Longitudinal Undetectable Molecular Residual Disease Defines Potentially Cured Population in Localized Non-Small Cell Lung Cancer
- PMID: 35543554
- PMCID: PMC9394392
- DOI: 10.1158/2159-8290.CD-21-1486
Longitudinal Undetectable Molecular Residual Disease Defines Potentially Cured Population in Localized Non-Small Cell Lung Cancer
Abstract
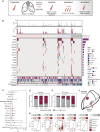
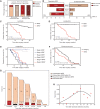
The efficacy and potential limitations of molecular residual disease (MRD) detection urgently need to be fully elucidated in a larger population of non-small cell lung cancer (NSCLC). We enrolled 261 patients with stages I to III NSCLC who underwent definitive surgery, and 913 peripheral blood samples were successfully detected by MRD assay. Within the population, only six patients (3.2%) with longitudinal undetectable MRD recurred, resulting in a negative predictive value of 96.8%. Longitudinal undetectable MRD may define the patients who were cured. The peak risk of developing detectable MRD was approximately 18 months after landmark detection. Correspondingly, the positive predictive value of longitudinal detectable MRD was 89.1%, with a median lead time of 3.4 months. However, brain-only recurrence was less commonly detected by MRD (n = 1/5, 20%). Further subgroup analyses revealed that patients with undetectable MRD might not benefit from adjuvant therapy. Together, these results expound the value of MRD in NSCLC.
Significance: This study confirms the prognostic value of MRD detection in patients with NSCLC after definitive surgery, especially in those with longitudinal undetectable MRD, which might represent the potentially cured population regardless of stage and adjuvant therapy. Moreover, the risk of developing detectable MRD decreased stepwise after 18 months since landmark detection. This article is highlighted in the In This Issue feature, p. 1599.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
-
When can we be confident of surgical cure with ctDNA?Nat Rev Clin Oncol. 2022 Sep;19(9):571-572. doi: 10.1038/s41571-022-00664-8. Nat Rev Clin Oncol. 2022. PMID: 35798966 No abstract available.
References
-
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. . Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9. - PubMed
-
- Burdett S, Rydzewska L, Tierney JF, Fisher DJ, PORT Meta-analysis Trialist Group. A closer look at the effects of postoperative radiotherapy by stage and nodal status: updated results of an individual participant data meta-analysis in non–small-cell lung cancer. Lung Cancer 2013;80:350–2. - PubMed
-
- Le Pechoux C, Pourel N, Barlesi F, Faivre-Finn C, Lerouge D, Zalcman G, et al. . LBA3_PR An international randomized trial, comparing postoperative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non–small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol 2020;31:S1178.
-
- Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. . Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med 2020;383:1711–23. - PubMed
-
- Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. . Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344–57. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

