Public health and budget impacts of switching from a trivalent to a quadrivalent inactivated influenza vaccine in Paraguay
- PMID: 35543602
- PMCID: PMC9302507
- DOI: 10.1080/21645515.2022.2069974
Public health and budget impacts of switching from a trivalent to a quadrivalent inactivated influenza vaccine in Paraguay
Abstract
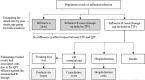
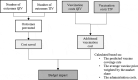
This study aimed to investigate the public health and economic benefit of using a quadrivalent influenza vaccine (QIV) instead of a trivalent influenza vaccine (TIV) in past seasons in Paraguay. The budget impact of switching from TIV to QIV in the Immunization Program was also evaluated. The adapted model includes two modules. The first compared retrospectively Health and Economic outcomes resulting from the use of QIV instead of TIV. The second forecast the spending and savings that would be associated with the switch from TIV to QIV. Our findings estimate that the switch from TIV to QIV during the seasons 2012 to 2017 could have prevented around 2,600 influenza cases, 67 hospitalizations and 10 deaths. An alternative scenario using standardized estimates of the burden of influenza showed that 234 influenza-related hospitalizations and 29 deaths could have been prevented. The estimated annual budget impact of a full switch from TIV to QIV was around USD1,6 million both from the payer and societal perspectives. Those results are mainly driven by vaccine prices and coverage rate. In sum, this manuscript describes how the use of QIV instead of TIV could have prevented influenza cases and subsequent complications that led to hospitalizations and deaths. This could have generated savings for the health system and society, offsetting part of the additional investment needed to switch from TIV to QIV.
Keywords: Paraguay; budget impact; influenza; public health impact; quadrivalent vaccine; trivalent vaccine; vaccine switch.
Conflict of interest statement
AA declares no conflict of interest. CMdC is employed in the Institute of Tropical Medicine, part of the National University of Paraguay, and received a grant from Sanofi Pasteur for the collection of the epidemiological data.
CV is employed in the Virology department of the Laboratorio Central de Salud Pública and received a grant from Sanofi Pasteur for the collection of the epidemiological data.CA, PMB, AP, HD, and JGL are employees Sanofi Pasteur, a company that manufactures and commercializes influenza vaccines.
LB is an employee of Creativ-Ceutical, which received funding from Sanofi Pasteur.
Figures

References
-
- Paget J, Spreeuwenberg P, Charu V, Taylor RJ, Iuliano AD, Bresee J, Simonsen L, Viboud C, Global Seasonal Influenza-associated Mortality Collaborator N, Teams* GLC . Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR project. J Glob Health. 2019;9:020421. doi: 10.7189/jogh.09.020421. - DOI - PMC - PubMed
-
- Bataglia S, Von Horoch M, Cabello A, Penayo E, Vázquez C. Carga de Influenza asociadas a hospitalizaciones y muertes en Paraguay, 2011-2015. Revista Paraguaya de Epidemiologia. 2017;4:25–33.
-
- WHO SAGE seasonal influenza vaccination recommendations during the COVID-19 pandemic; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
