Automated preclinical detection of mechanical pain hypersensitivity and analgesia
- PMID: 35543646
- PMCID: PMC9649838
- DOI: 10.1097/j.pain.0000000000002680
Automated preclinical detection of mechanical pain hypersensitivity and analgesia
Abstract
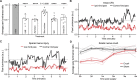
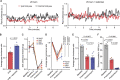
The lack of sensitive and robust behavioral assessments of pain in preclinical models has been a major limitation for both pain research and the development of novel analgesics. Here, we demonstrate a novel data acquisition and analysis platform that provides automated, quantitative, and objective measures of naturalistic rodent behavior in an observer-independent and unbiased fashion. The technology records freely behaving mice, in the dark, over extended periods for continuous acquisition of 2 parallel video data streams: (1) near-infrared frustrated total internal reflection for detecting the degree, force, and timing of surface contact and (2) simultaneous ongoing video graphing of whole-body pose. Using machine vision and machine learning, we automatically extract and quantify behavioral features from these data to reveal moment-by-moment changes that capture the internal pain state of rodents in multiple pain models. We show that these voluntary pain-related behaviors are reversible by analgesics and that analgesia can be automatically and objectively differentiated from sedation. Finally, we used this approach to generate a paw luminance ratio measure that is sensitive in capturing dynamic mechanical hypersensitivity over a period and scalable for high-throughput preclinical analgesic efficacy assessment.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
D. P. Roberson, C. J. Woolf, A. B. Wiltschko, L. B. Barrett, and S. R. Datta have issued patents on the data acquisition technology. D. P. Roberson, L. B. Barrett, and C. J. Woolf have a financial relationship with Blackbox Bio, the company which has licensed the patent on the technology from Boston Children's Hospital. D. P. Roberson is also now an employee of Blackbox Bio.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. PAIN 1995;60:91–102. - PubMed
-
- Berridge KC, Whishaw IQ. Cortex, striatum and cerebellum: control of serial order in a grooming sequence. Exp Brain Res 1992;90:275–90. - PubMed
-
- Berryman ER, Harris RL, Moalli M, Bagi CM. DigigaitTM quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact 2009;9:89–98. - PubMed
-
- Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol 2000;428:45–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

